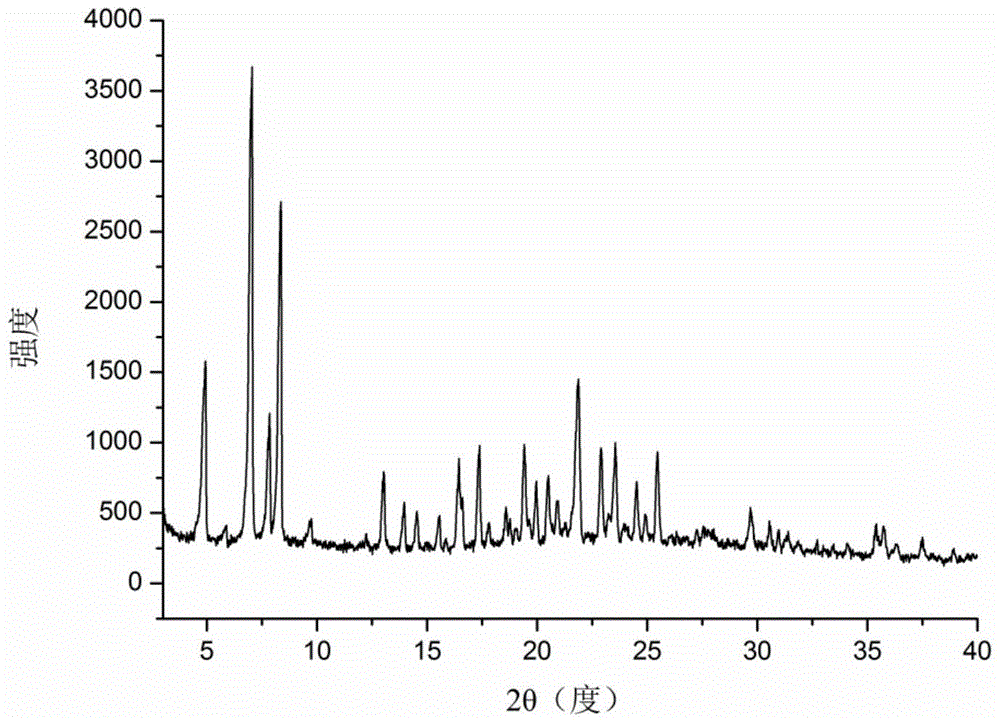
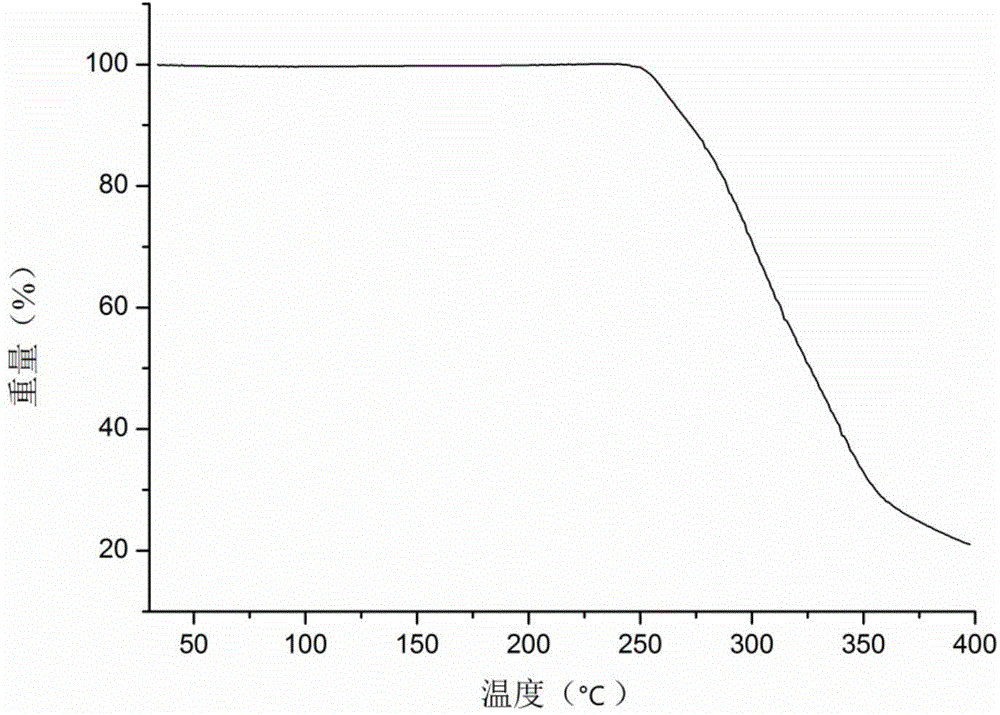
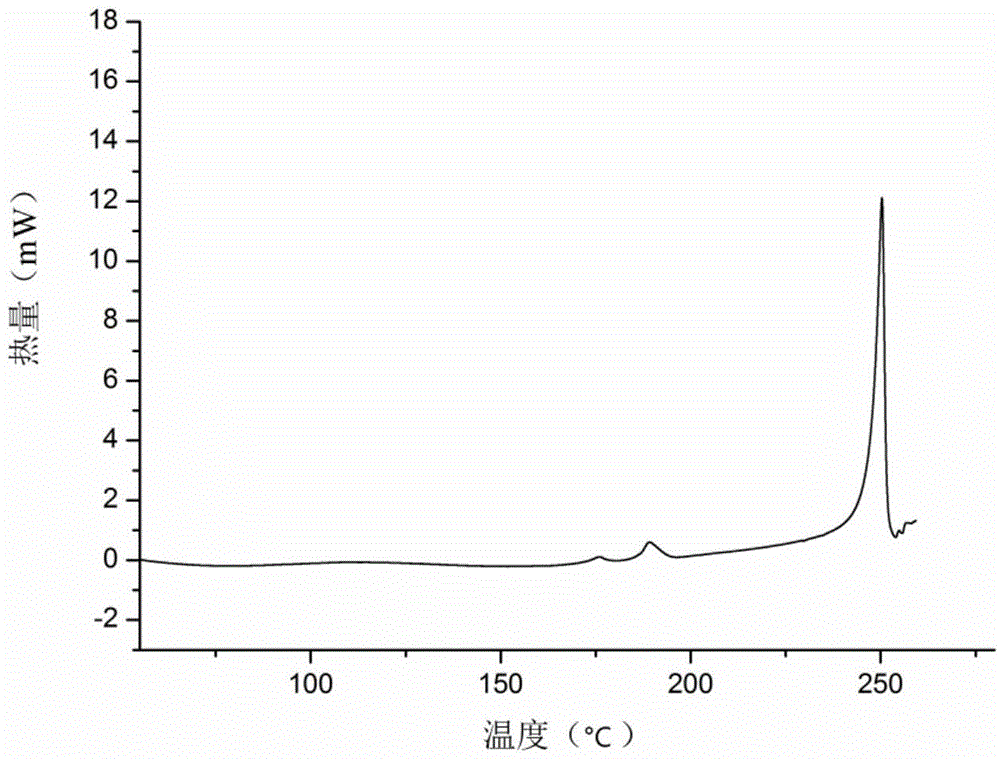
Icariin H1 crystal form, as well as production method, pharmaceutical composition and application thereof
A technology of icariin and crystal form, applied in the field of medicinal chemistry, can solve the problems of poor water solubility of icariin, poor oral absorption, limited clinical application, etc., and achieves good reproducibility, good absorption and utilization, and oral absorption. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 30 mg of icariin in a bottle, add 3 mL of methanol, sonicate to dissolve and become supersaturated, suspend at room temperature for 3 days, centrifuge the suspension, discard the supernatant, and centrifuge The solid was vacuum-dried at 50°C to obtain icariin H1 crystal form.
Embodiment 2
[0039] Take 30mg of icariin in a bottle, add 3mL of absolute ethanol, ultrasonicate to dissolve and become supersaturated, suspend at room temperature for 3 days, centrifuge the suspension, discard the supernatant, and centrifuge The resulting solid was vacuum-dried at 50° C. to obtain icariin H1 crystal form.
Embodiment 3
[0041]Take 30 mg of icariin in a bottle, add 3 mL of n-propanol with a mass concentration of 70%, ultrasonically dissolve it and make it supersaturated, suspend at room temperature for 3 days, centrifuge the suspension, and discard For the supernatant, the centrifuged solid was evaporated to dryness at 100°C to obtain icariin H1 crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com