Hematopoietic stem cell culture medium and its application and stem cell cultivation method based on hematopoietic stem cell culture medium
A technology for hematopoietic stem cells and culture medium, applied in cell culture medium, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of slow proliferation rate and low passage number of hematopoietic stem cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
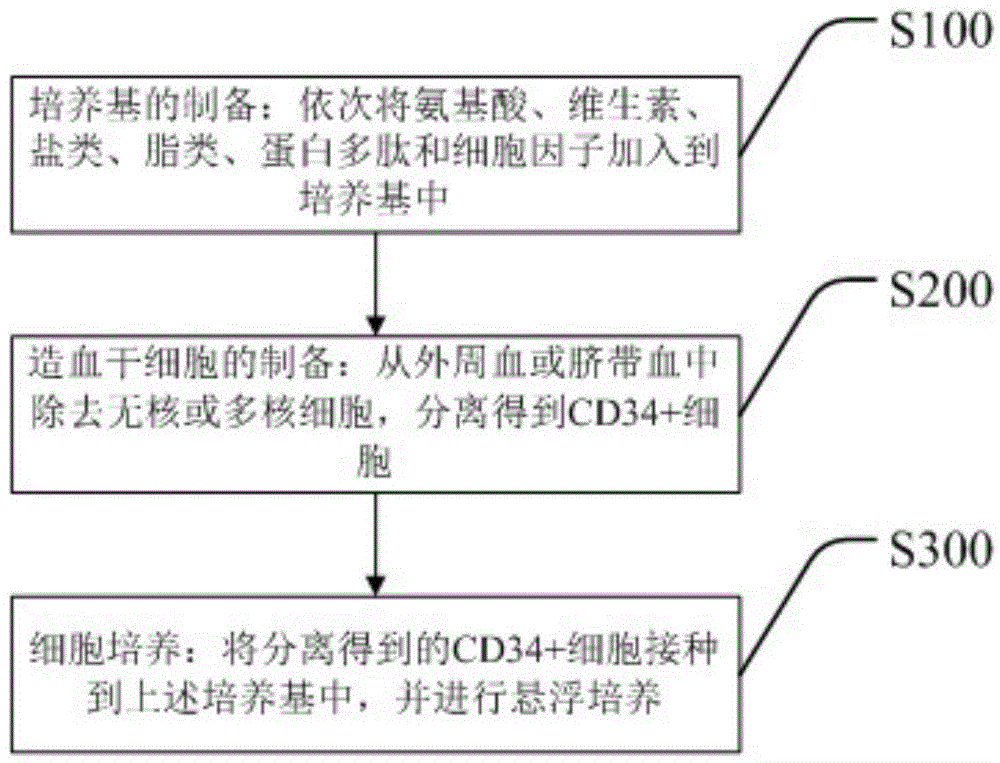
[0127] S100. Preparation of culture medium: adding amino acids, vitamins, salts, lipids, protein polypeptides and cytokines to the culture medium in sequence. Of course, for step S100, the substances to be added depend on the actual situation. For example, plant or animal cell extracts can be added. The specific process is to undergo high-temperature extraction treatment, and high-speed centrifugation to remove large insoluble particles, and then Filter with 0.22 μm and 0.1 μm filter membranes successively, inactivate and remove insoluble precipitates and potential pathogens, and then add to the culture medium. In addition, trace elements or small organic molecules can also be added.
[0128] S200. Preparation of hematopoietic stem cells: removing anucleated or multinucleated cells from peripheral blood or umbilical cord blood, and isolating CD34+ cells. Specifically, a certain amount of serum or plasma is added to the culture flask, and anucleated or multinucleated cells are...
Embodiment 1
[0143] Example 1. Proliferation of hematopoietic stem cells
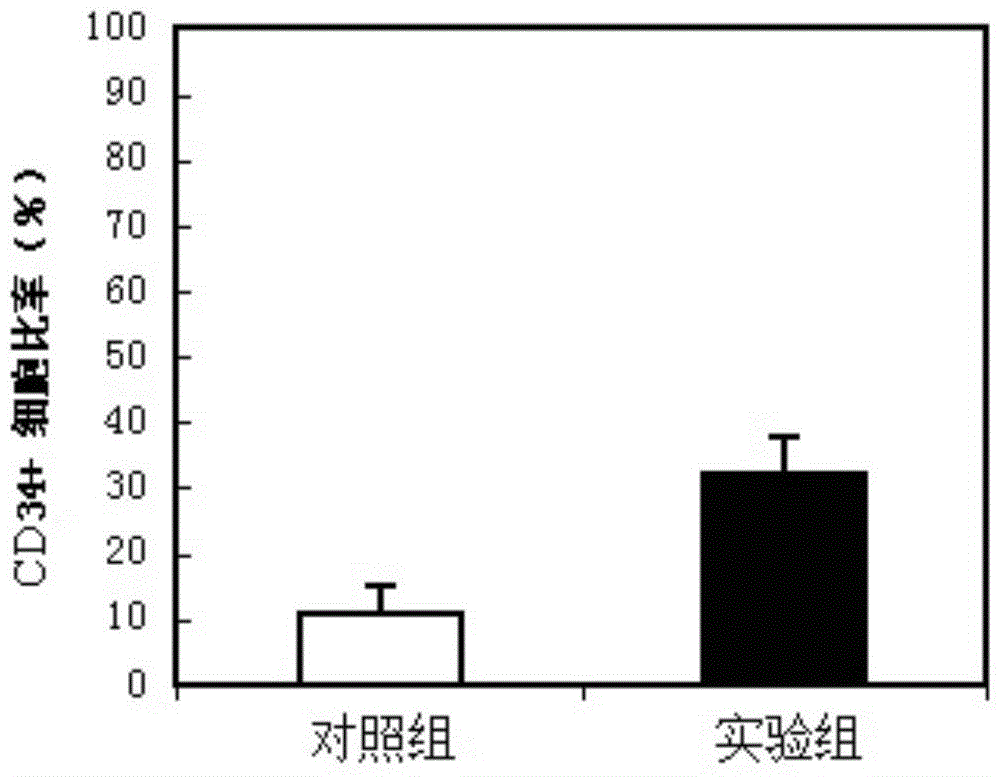
[0144] In order to check the influence of the hematopoietic stem cell culture medium of the present invention on the expansion speed of hematopoietic stem cells, the peripheral blood after 4 days of G-CSF mobilization was selected to prepare mononuclear cells with the Ficol method, and the obtained monocytes were subjected to in vitro cell culture experiments. cells by 10 4 Add control culture medium (control group) and the hematopoietic stem cell culture medium (experimental group) of the present invention after inoculation and cultivate respectively (37 degrees Celsius, 5% CO 2 ), 4-7 days later, hematopoietic stem cells characterized by CD34+ were sorted by anti-CD34 antibody flow cytometry, and the number of CD34+ cells and their ratio to the total cells were calculated, and the total number of cells was determined by the MTT method.
[0145] The results show that under the same conditions, the CD34+ cell ratio...
Embodiment 2
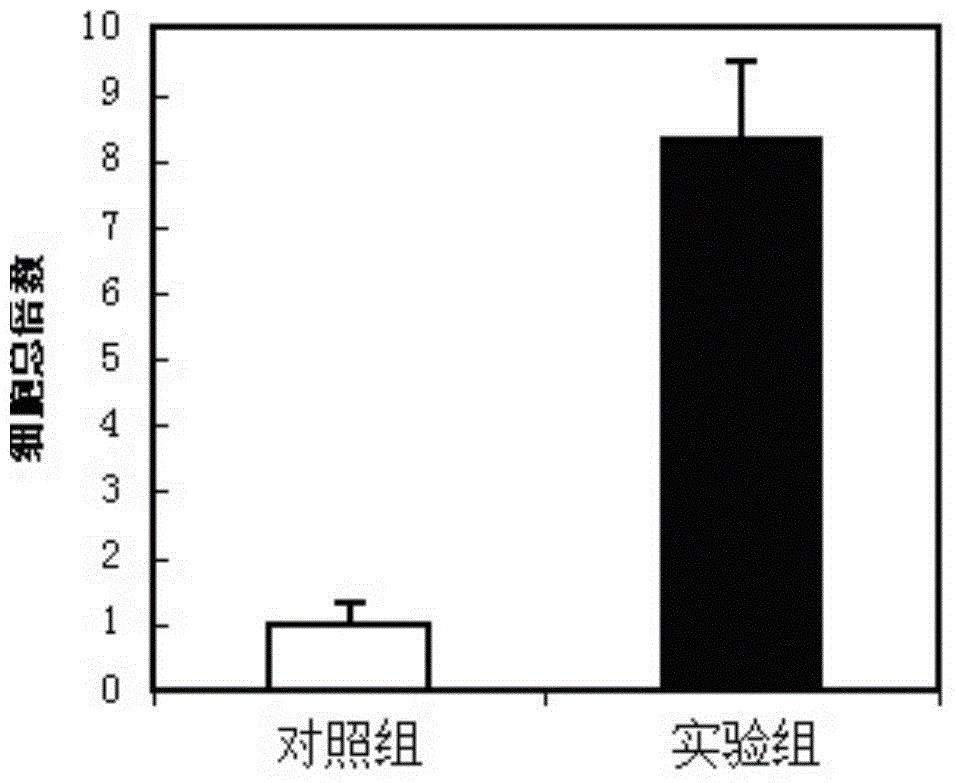
[0146] In order to check the influence of the hematopoietic stem cell culture medium of the present invention on the telomere length of hematopoietic stem cells, after injecting mice with G-CSF and mobilizing them for 4 days, get peripheral blood to prepare mononuclear cells by the Ficol method, and further use conjugated anti- Magnetic beads of CD34 antibody enrich CD34+ cells. Divide CD34+ cells by 10 4 Inoculated into the hematopoietic stem cell medium (experimental group) of the present invention and conventional control medium (control group), suspension culture (37 degrees Celsius, 5% CO 2 ) After 1 week, the telomere length of the cultured cells was measured according to the fluorescent quantitative PCR method described in the literature (Journal of Clinical and Experimental Medicine 2008(7):14). The results showed that the telomere length of the experimental group was longer than that of the control group 8% (see Figure 4 ); data are mean±SEM of 12 independent exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com