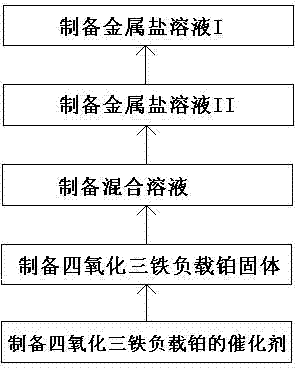
Preparation method and application of platinum-loaded ferriferrous oxide catalyst
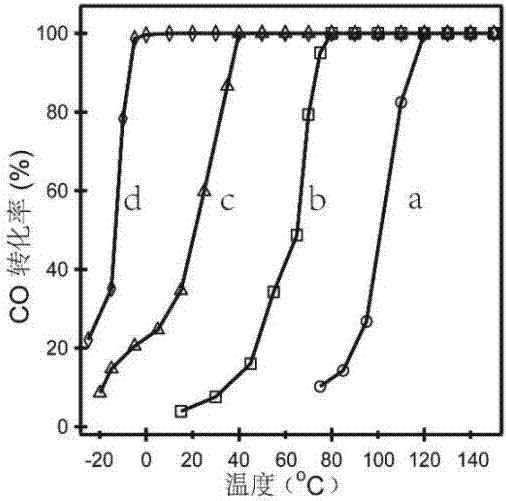
A technology of ferroferric oxide and catalysts, applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of low activity of catalytic CO oxidation and the amount of precious metals Large demand, complex preparation process and other problems, to achieve good catalytic CO oxidation activity and stability, not harsh reaction conditions, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 (see figure 1 )
[0050] A preparation method of a platinum-supported catalyst comprising ferric iron tetroxide, comprising the following steps:
[0051] (1) Preparation of metal salt solution I
[0052] Add 2.78g of ferrous sulfate solid (analytical pure produced by Sinopharm Reagent Co., Ltd., quality purity ≥ 99%) in 10ml of deionized water, stir at room temperature for 10min, and dissolve to obtain ferrous sulfate solution.
[0053] (2) Preparation of metal salt solution II
[0054] Add 2.4 g of potassium hydroxide (analytical pure produced by Sinopharm Reagent Co., Ltd., quality purity ≥ 99%) to 150 ml of deionized water, and stir at 90 °C for at least 10 min to obtain a potassium hydroxide solution.
[0055] (3) Preparation of mixed solution
[0056] Add the potassium hydroxide solution obtained in step (2) into the ferrous sulfate solution obtained in step (1) until the ferrous sulfate solution is completely precipitated, and continue stirring at ...
Embodiment 2
[0064] A preparation method of a platinum-supported catalyst comprising ferric iron tetroxide, comprising the following steps:
[0065] (1) Preparation of metal salt solution I
[0066] Add 2.78g of ferrous sulfate solid (analytical pure produced by Sinopharm Reagent Co., Ltd., quality purity ≥ 99%) in 10ml of deionized water, continue to add 3ml of chloroplatinic acid solution with a concentration of 10g / L, stir at room temperature for 10min, and get ferrous sulfate solution.
[0067] (2) Prepare metal salt solution II (same as Example 1).
[0068] Add 2.4 g of potassium hydroxide (analytical pure produced by Sinopharm Reagent Co., Ltd., mass purity ≥ 99%) to the solution obtained in 150 ml of deionized water, and stir at 90 °C for at least 10 min to obtain a potassium hydroxide solution.
[0069] (3) Preparation of mixed solution
[0070] Add the potassium hydroxide solution obtained in step (2) into the ferrous sulfate solution obtained in step (1), until the ferrous sul...
Embodiment 3
[0078] A preparation method of a platinum-supported catalyst of ferric iron tetroxide, the basic steps of which are the same as in Example 2. Embodiment 3 is different in that:
[0079] (1) Add 1.3g of ferrous chloride (analytical pure produced by Sinopharm Reagent Co., Ltd., quality purity ≥ 99%) in 10ml of deionized water, continue to add 9ml of sodium chloroplatinate solution with a concentration of 10g / L, and stir at room temperature for 10min , after dissolving, ferrous chloride and platinum salt solution are obtained.
[0080] Added in step (2) is sodium hydroxide;
[0081] A platinum catalyst supported by ferric iron tetroxide with a loading of 2.5wt% was obtained.
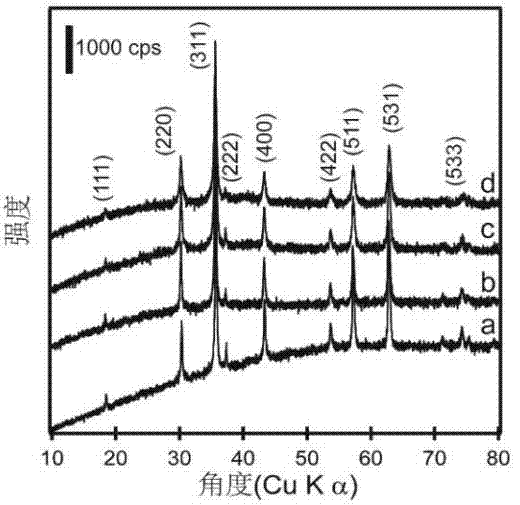
[0082] The catalyst of ferric iron tetroxide supported platinum prepared in embodiment 3 is detected by X-ray powder diffractometer:
[0083] X-ray diffraction (XRD) pattern (see figure 2 The results in c) show that the ferric iron tetroxide-supported platinum catalyst prepared in Example 3 has good cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com