Cefmetazole sodium compound and preparation method thereof
A technology for cefmetazole sodium and compound, which is applied in the field of medicinal chemistry, can solve the problems of high impurities, expensive equipment, and energy utilization rate of only 2%, and achieves the effects of uniform particle size distribution, solvent-friendly, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Step 1: join the cefmetazole sodium of 20kg in the NMP of 60L, heat up and stir and make it dissolve, press into the crystallization tank of aseptic zone through the filter of 0.22 μm;
[0059] Step 2: Warm up the solution obtained in step 1 to 40°C; add 150 L of isopropanol that has been filtered through a 0.22 μm filter membrane at a stirring speed of 200 r / min at a speed of 340 ml / min; dropwise addition is complete Then add 150 L of the remaining isopropanol that is also sterile-filtered at a rate of 100 ml / min;
[0060] Step 3: At a stirring speed of 125r / min, the mixed solution of methyl tert-butyl ether and acetone (wherein methyl tert-butyl ether: acetone = 2:1) filtered through a 0.22μm filter membrane was mixed with 200ml / Add 720L dropwise at a speed of min. After the dropwise addition is completed, cool down to 25°C at a rate of 5°C / h and keep it warm for 4 hours to grow crystals;
[0061] Step 4: Cool the solution to 3°C and keep it warm for 2 hours, then ...
Embodiment 2
[0067] Step 1: Add 16kg of cefmetazole sodium into 32L of NMP, heat up and stir to dissolve;
[0068] Step 2: After heating the solution obtained in step 1 to 45°C, start to drop 48L of isopropanol at a rate of 300ml / min at a stirring speed of 250r / min, and then add dropwise at a rate of 100ml / min after the addition is completed The remaining 48L of isopropanol;
[0069] Step 3: At a stirring speed of 150r / min, add 320L of a mixed solution of methyl tert-butyl ether and acetone (wherein methyl tert-butyl ether: acetone = 1:1) dropwise at a speed of 200ml / min. After the dropwise addition, the temperature was lowered to 27°C at a rate of 5°C / h, and then kept for 4 hours for crystal growth;
[0070] Step 4: Cool the solution to 5°C and keep it warm for 1 hour, then suction filter; the filter cake was dried under reduced pressure at 40°C to constant weight to obtain cefmetazole sodium crystals with a yield of 86.2% and a purity of 99.7% by HPLC.
Embodiment 3
[0073] Step 1: Add 15kg of Cefmetazole Sodium into 60L of NMP, heat up and stir to dissolve it; Step 2: After the solution obtained in Step 1 is warmed up to 35°C, start stirring at 400ml / min at a stirring speed of 150r / min. Add 210L of isopropanol dropwise at a speed of 1 min, and then add the remaining 210L of isopropanol dropwise at a speed of 100ml / min;
[0074] Step 3: At a stirring speed of 100r / min, add 900L of a mixed solution of methyl tert-butyl ether and acetone (wherein methyl tert-butyl ether: acetone = 3:1) dropwise at a speed of 200ml / min. After the dropwise addition is completed, cool down to 25°C at a rate of 5°C / h and keep it warm for 5 hours to grow crystals;
[0075] Step 4: Cool the solution to 0°C and keep it warm for 2 hours, then suction filter; the filter cake was dried under reduced pressure at 45°C to constant weight to obtain cefmetazole sodium crystals with a yield of 88.4% and a purity of 99.5% by HPLC.
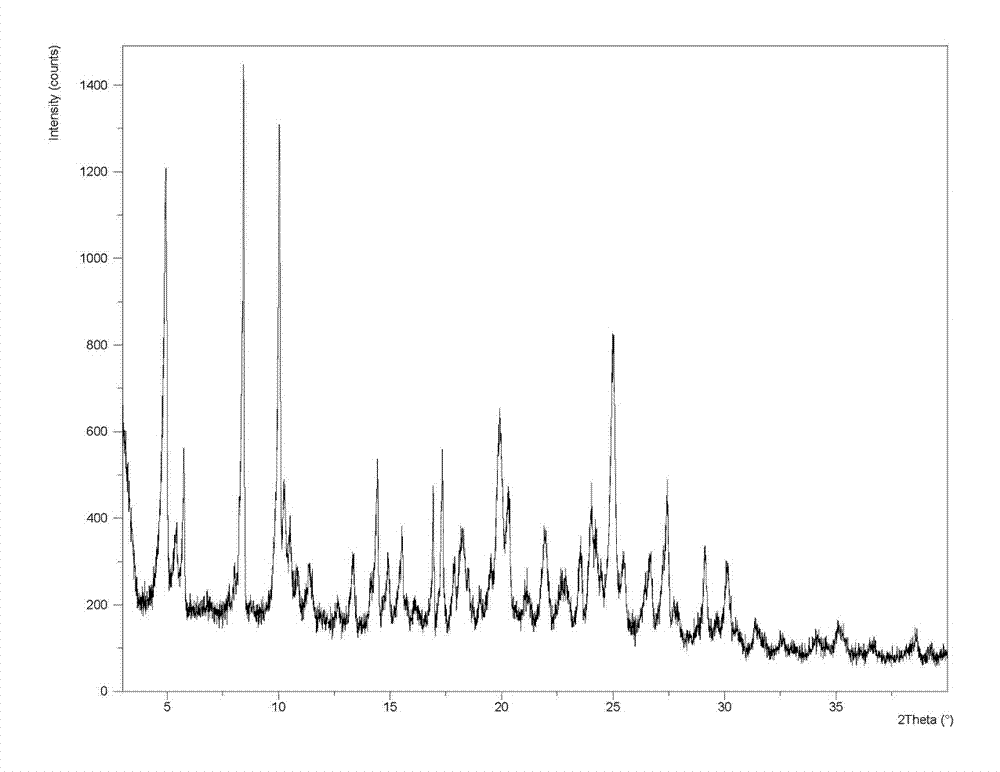
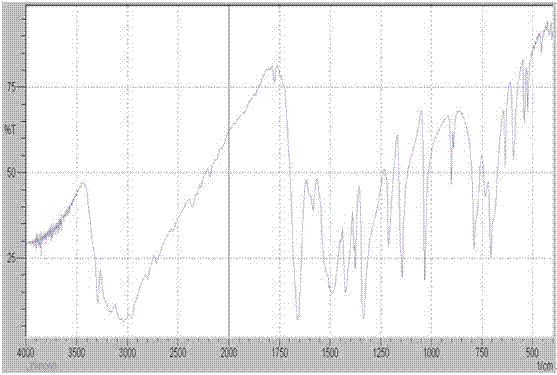
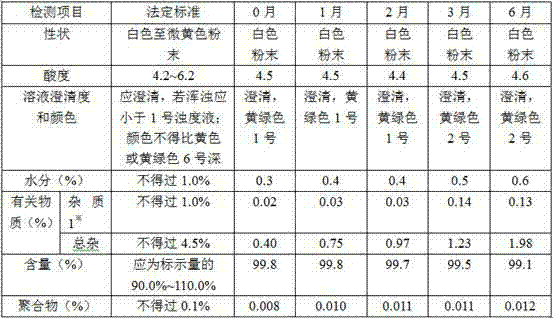
[0076] The X-ray powder diffraction patte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com