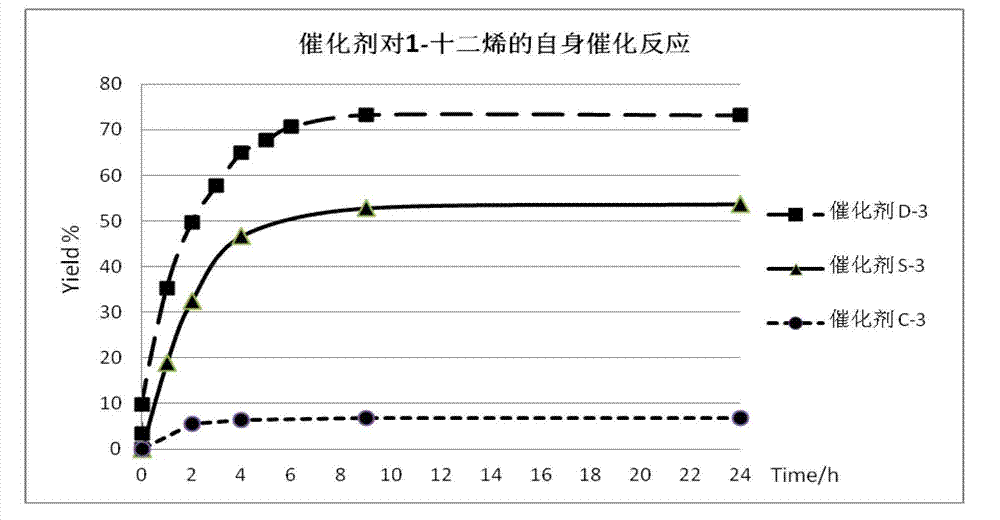
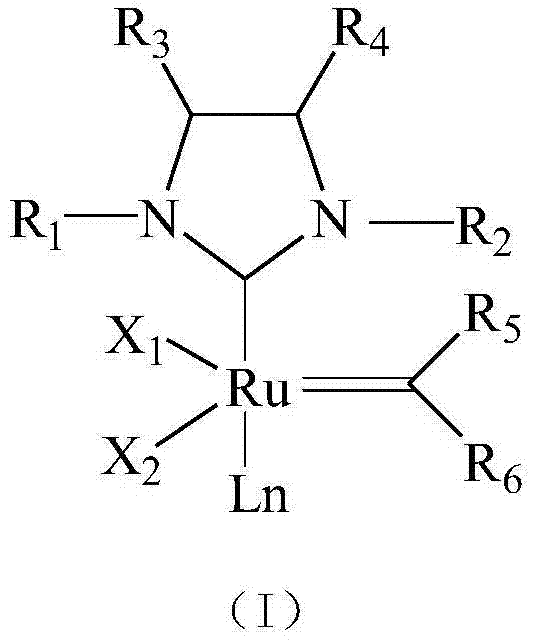
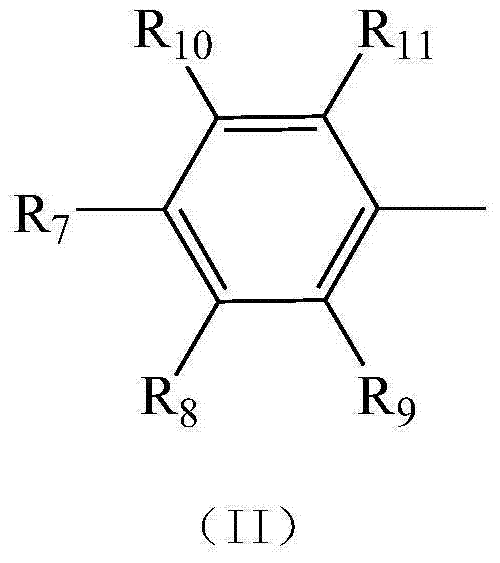
Novel N-heterocyclic carbene ruthenium catalyst containing electron donating group and preparation method thereof
An aryl, alkyl technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Synthesis of ligands containing double electron-pushing groups 1,3-bis(4-isopropoxy-2,6-dimethylphenyl)-imidazole Onium chloride
[0093] Synthesis of 4-isopropoxy-2,6-dimethylaniline: 4-amino-3,5-dimethylphenol (1.27 g, 10 mmol) and 60% sodium hydride ( 0.60g, 15mmol) was added into the dimethylformamide (DMF, 15ml) solvent dried through 4A molecular sieves, exothermic phenomenon occurred, then potassium carbonate (0.34g, 2.46mmol) and bromoisopropane (2.67g , 19mmol) in a 50ml two-necked bottle. Under the protection of argon, a reflux condensing device is equipped, and the reaction bottle reacts for 22 hours under stirring at 50 degrees Celsius, and judges whether the end point of the reaction is reached with a TLC plate, and after 4 hours of reaction, bromoisopropane (0.85 g) is added, and the reaction solution From light brown to dark brown, then overnight reaction, and finally the reaction was completed and left to cool. Filter the reaction solution a...
Embodiment 2
[0099] Example 2 Synthesis of a ligand containing a single electron pusher group 1-[(1,3,5-trimethylphenyl) base]-3-([4-isopropoxy -2,6-Dimethylphenyl)-yl]-imidazolium chloride
[0100] Adopt the synthetic method and route of above-mentioned embodiment 1, use two different aromatic amines, through condensation, reduction, Different bisaryl-ethylenediimine, ethylenediamine and imidazolium chlorides can be prepared conveniently by reaction and separation such as ring formation. Tool The specific steps and operations are as follows:
[0101] Synthesis of 1-(1,3,5-trimethylphenyl)-2-(4-isopropoxy-2,6-dimethylphenyl)-ethylenediimine: add 4- Isopropoxy-2,6-dimethylaniline (4g), glyoxal (8g) and trimethylaniline (4g), then add 50% isopropanol / water (50ml) and 13% acetic acid / Isopropanol (10 drops), stirred and reacted at room temperature for 4h, the reaction solution gradually turned into a yellow solution, and the TLC plate (10% ethyl acetate / n-hexane) was used to judge ...
Embodiment 3
[0107] Example 3 Synthesis of catalyst ligands without substituents at the 3 and 5 positions of the benzene ring
[0108] The starting material 4-methoxyaniline is a commercial product. Its intermediates: 1,2-bis(4-methoxyphenyl)-ethylenediimine, 1,2-bis(4-methoxyphenyl)-ethylenediamine, 1,3-bis(4 -Methoxyphenyl) base)-imidazolium chloride, the synthetic method and route of the ligand of the above-mentioned embodiment 1 can be adopted. The synthesized intermediates were identified by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com