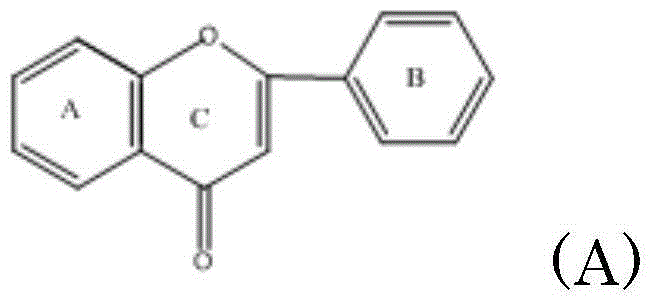
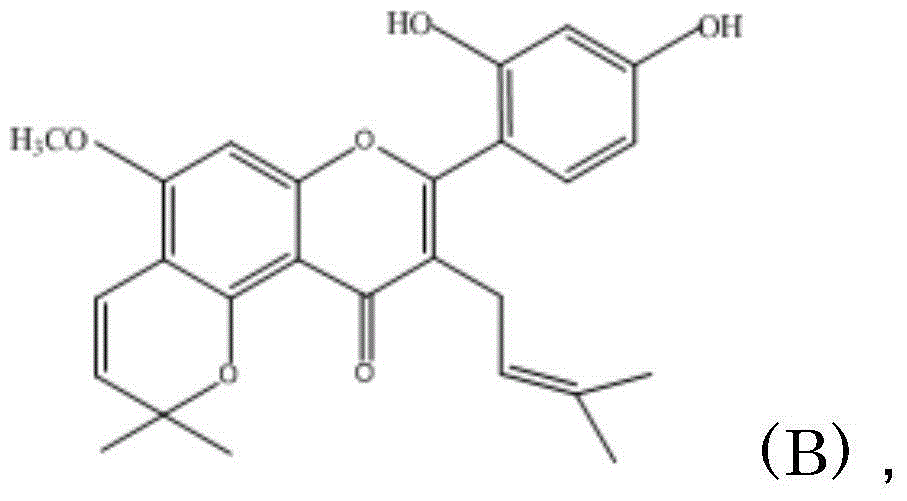
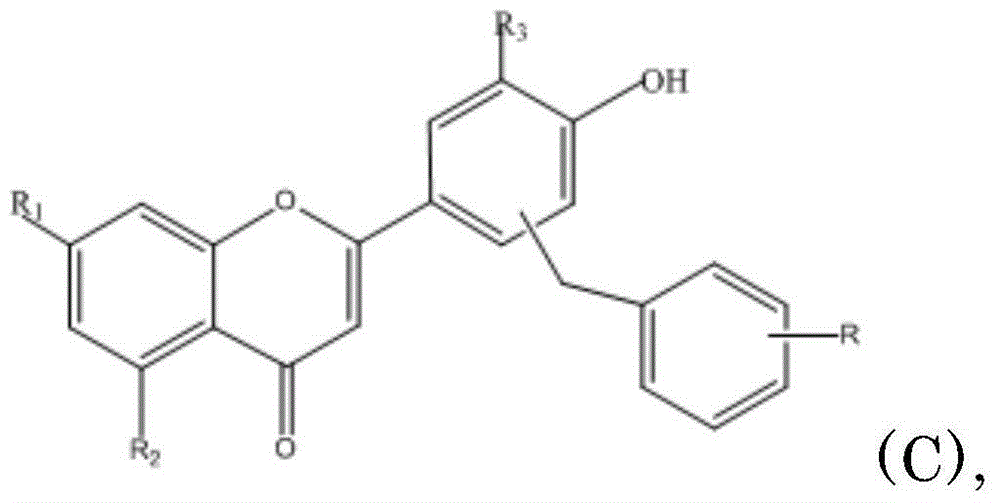
Prodrug of flavonoids and application of prodrug
A technology of compounds and prodrugs, applied in the field of prodrugs of flavonoids, can solve the problems of low bioavailability, poor solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of Alcoradine
[0065] Acradine, also known as icariin, is extracted and separated from the traditional Chinese medicine Epimedium, and its structure is shown in the following formula (G).
[0066]
[0067] The preparation method of icariin is disclosed in the patent whose publication number is CN101302548. In the method, icariin is used as a raw material, hydrolyzed by β-glucosidase, the precipitate obtained by centrifuging the hydrolyzed product is dissolved in acetone, and the supernatant is obtained by centrifuging and filtering. The supernatant obtained by centrifugation is then recrystallized with water to obtain pure icariin, which is in the form of yellow powder crystals. The icariin in the present invention was purchased from Shaanxi Jiahe Plant Chemical Co., Ltd., with a purity of 90%.
Embodiment 2
[0069] 3,5-Dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-chromen-4-one)phosphonic acid di Synthesis and identification of sodium ester (formula I compound), synthetic route is as follows:
[0070]
[0071] Compound H 3,5-dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxybenzene Preparation of diethyl)-7-(4H-benzopyran-4-one)phosphate
[0072] Compound G 3,5,7-trihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyran-4 -ketone) (7.4g, 20mmol, 1.0eq) was dissolved in 100ml of anhydrous tetrahydrofuran (THF), added DIPEA (15ml, 83.7mmol, 4.2eq), stirred well, added DMAP (0.24g, 2mmol, 10%mol), Cool down to -10°C, slowly add CCl dropwise 4 (3ml, 31mmol, 1.5eq), 10ml of anhydrous THF solution of diethyl phosphite (3.32g, 2.4mmol, 1.2eq), slowly rise to room temperature for reaction, thin-layer chromatography (silica gel plate loading material, developing agent is acetic acid Ethyl ester and petroleum ether 1:2, 254nm ultraviolet lamp color) monitor un...
Embodiment 3
[0094] 3,5-Dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzene Preparation, synthesis and identification of pyran-4-one) sodium sulfate
[0095]
[0096] Compound G (3.68g, 10mmol, 1.0eq) was dissolved in 40ml of dry pyridine / dimethylformamide (DMF) (1:1 v / v), slowly added in batches to pyridine sulfur trioxide complex (C 5 h 5 NSO 3 ) (4.8g, 30mmol, 6.0eq), after the addition is complete, keep away from light, heat and stir the reaction at 50-60°C, thin-layer chromatography (silica gel plate loading material, developing agent is ethyl acetate and petroleum ether 2:1, 254nm UV Light color) Monitor until compound G disappears completely, the reaction solution naturally cools down to room temperature, cools down to 0°C in an ice bath, slowly adds 30ml of water under stirring and stirs for 30 minutes, adjusts the pH value to 13 with 1mol / l NaOH, evaporates to dryness and removes Pyridine and DMF, add 40ml of water, add dropwise 1mol / l sulfuric acid to acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com