Small crystal ZSM-5, its synthesis and use
A ZSM-5, crystal technology, applied in the field of small crystal size ZSM-5, can solve difficult problems such as processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation of the C4 diquaternary salt (diquat) of N-pentylpyrrolidine
[0075] N-pentylpyrrolidine was initially prepared by reductive amination of valeraldehyde with pyrrolidine according to the following procedure.
[0076] Place 500 mL of tetrahydrofuran (THF) in a 1-L suction filter flask equipped with nitrogen flow. 31.9 g valeraldehyde (0.37 mol) and then 24.9 g pyrrolidine (0.35 mol) were mixed into THF. Turn off the nitrogen flow, then add 100 g of sodium triacetoxyborohydride powder to the solution in 5-10 g increments. During the addition, vigorous stirring was used to ensure that the powder did not clump at the bottom of the flask and to ensure efficient mixing of the suspension. After each addition of sodium triacetoxyborohydride powder, allow sufficient time to form a homogeneous slurry before the next addition of the powder. Once all the powder addition is complete, the nitrogen flow is then turned on. After 2 days, the product was ...
Embodiment 2
[0079] Embodiment 2: Adopt the C4 diquaternary salt of N-pentylpyrrolidine to synthesize ZSM-5
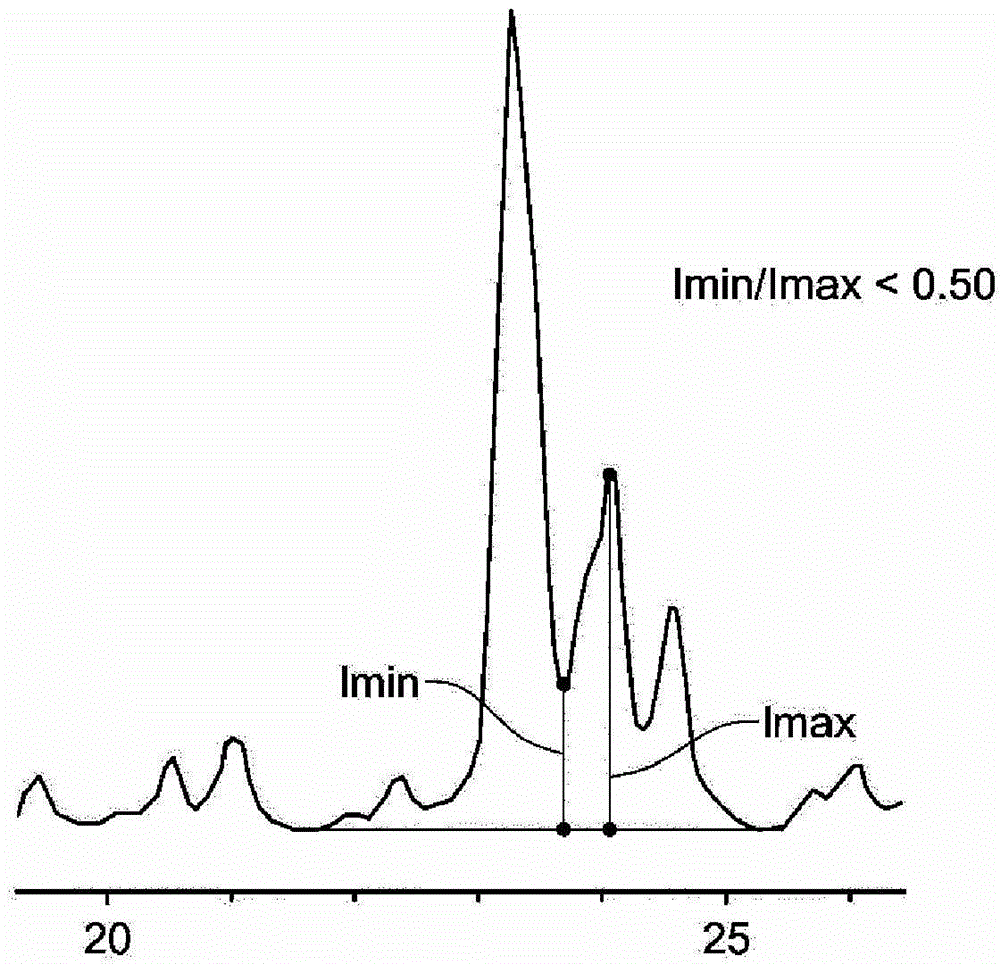
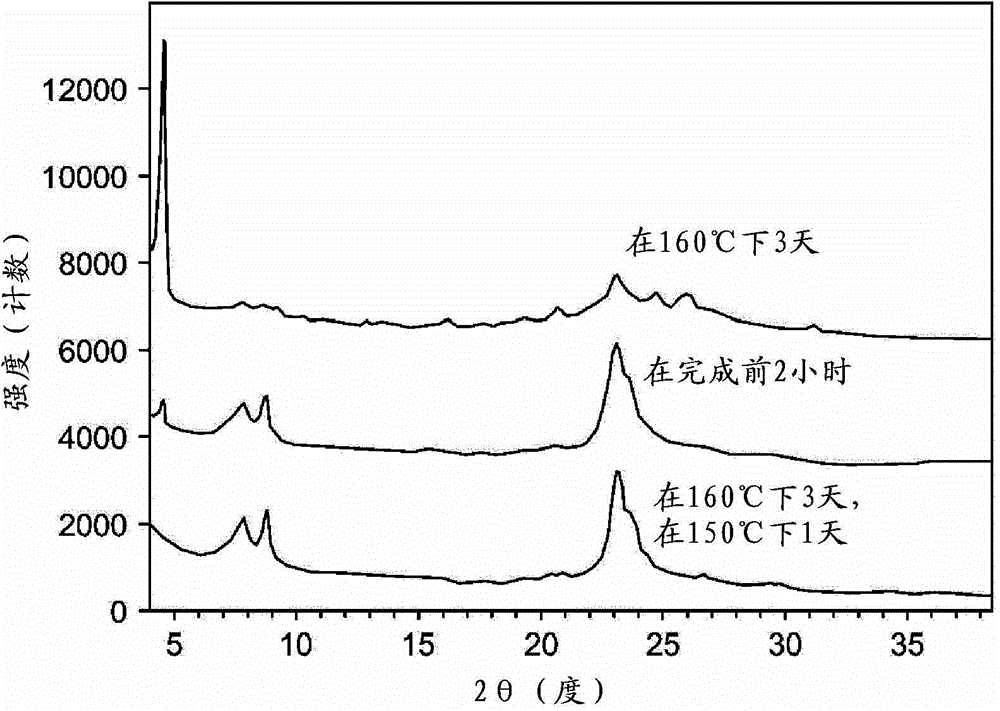
[0080] This synthesis was carried out using the hydroxide exchanged form of the C4 diquaternary salt of N-pentylpyrrolidine (prepared from the reaction of 1,4-dibromobutane and N-pentylpyrrolidine). The diquaternary salt solution of 55.1g ([OH - ] = 1.02 mmol / g) was mixed with 49.1 g of 1N KOH and 22.4 g of deionized water. 1.59 g of fumed alumina was added, and the mixture was mixed well to produce a homogeneous suspension. Then 41.6 g of Ludox AS-40 colloidal silicon dioxide were mixed into the suspension. The mixture was placed inside a Teflon liner and sealed in a 300-mL capacity Parr autoclave equipped with an overhead stirrer. The overhead stirrer was set at 150 rpm. The gel was heated to 160°C during 4 hours. After 3 days, samples were removed from the autoclave (on-line) and subjected to powder X-ray diffraction (XRD) analysis. The product is mainly a lamellar phase (...
Embodiment 3
[0084] Embodiment 3: Adopt the C5 diquaternary salt of N-pentylpyrrolidine to synthesize ZSM-5
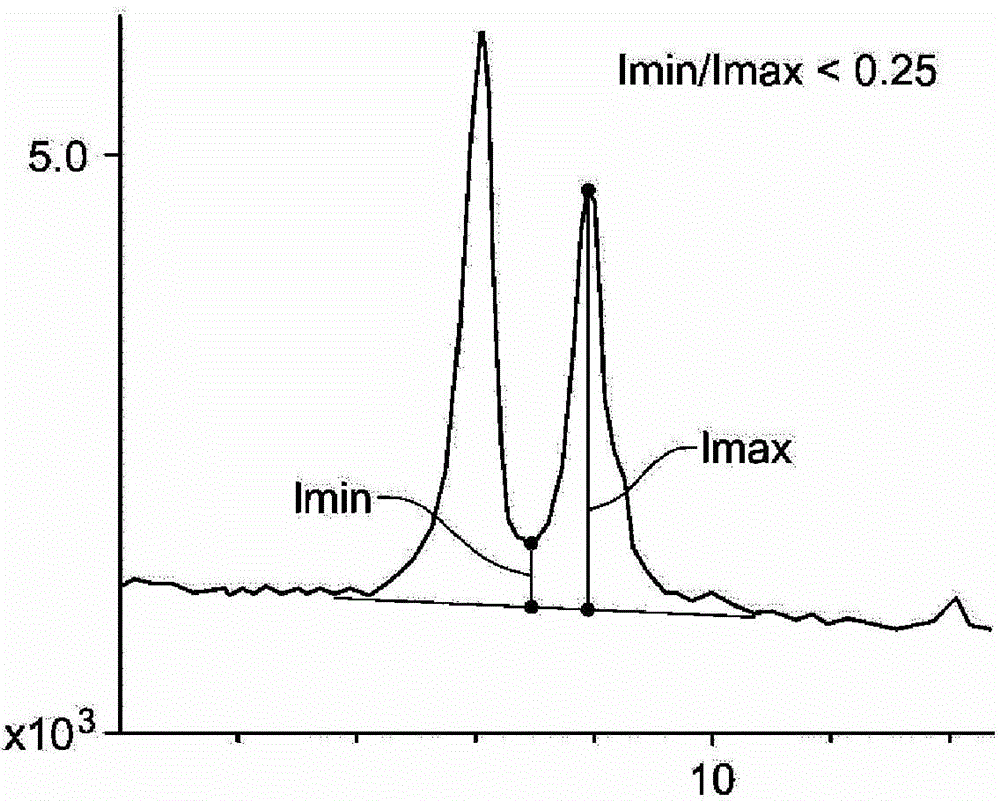
[0085] This synthesis was performed using the hydroxide exchanged form of the C5 diquaternary salt of N-pentylpyrrolidine (prepared from the reaction of 1,5-dibromopentane and N-pentylpyrrolidine). The diquaternary salt solution of 87.2g ([OH -]=0.58mmol / g) was mixed with 44.03g 1N KOH. 1.05 g of fumed alumina was added, and the mixture was mixed well to produce a homogeneous suspension. 37.7 g of Ludox AS-40 colloidal silicon dioxide were then mixed into the suspension. The mixture was placed inside a Teflon liner and sealed in a 300-mL capacity Parr autoclave equipped with an overhead stirrer. The overhead stirrer was set at 150 rpm. The gel was heated to 160°C during 4 hours. After 24 hours, samples were removed from the autoclave (on-line) and subjected to powder XRD analysis. The product was pure (by XRD) ultra-small ZSM-5. Figure 5 The powder XRD pattern of the produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| External area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Internal surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap