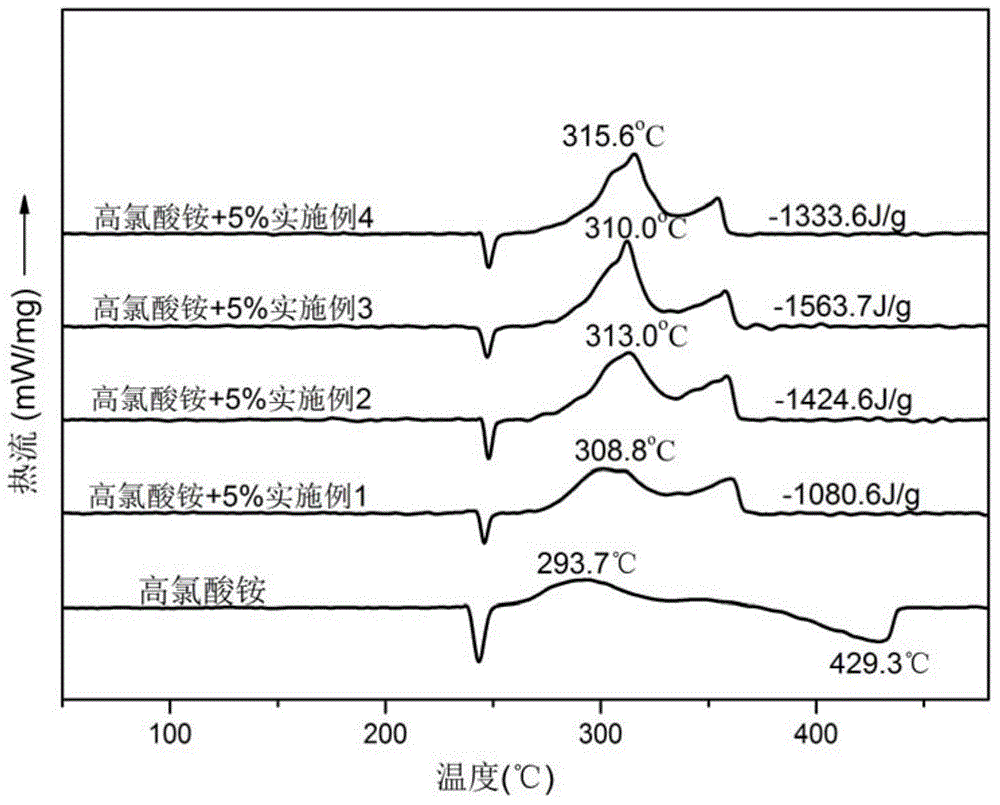
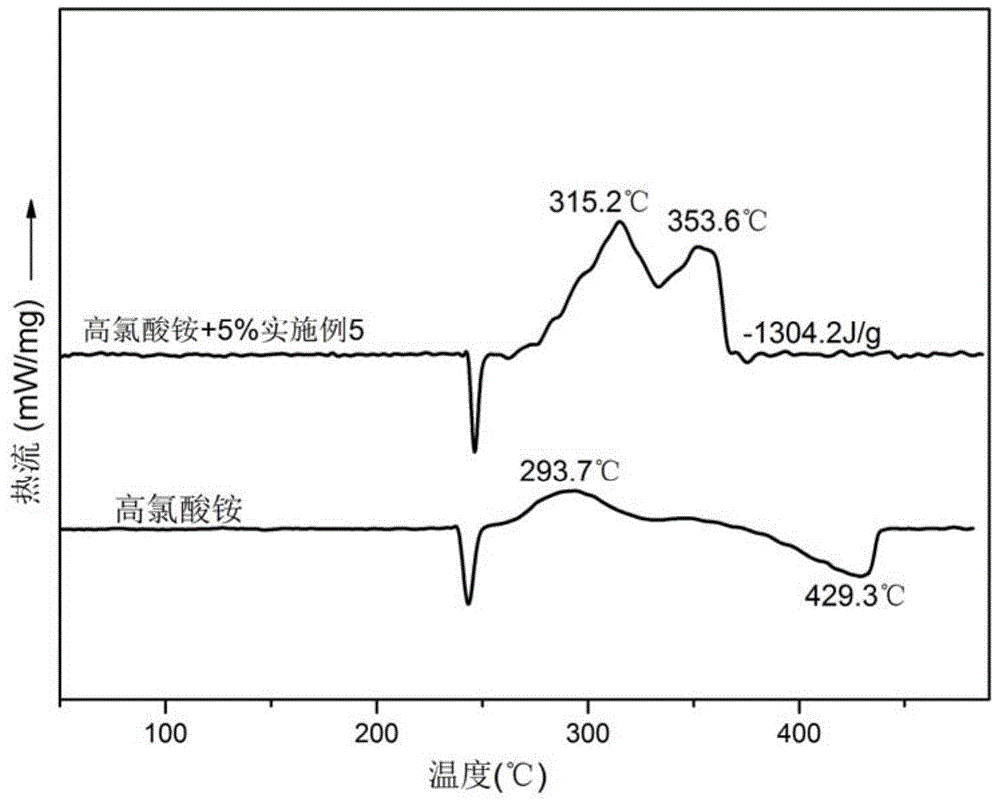
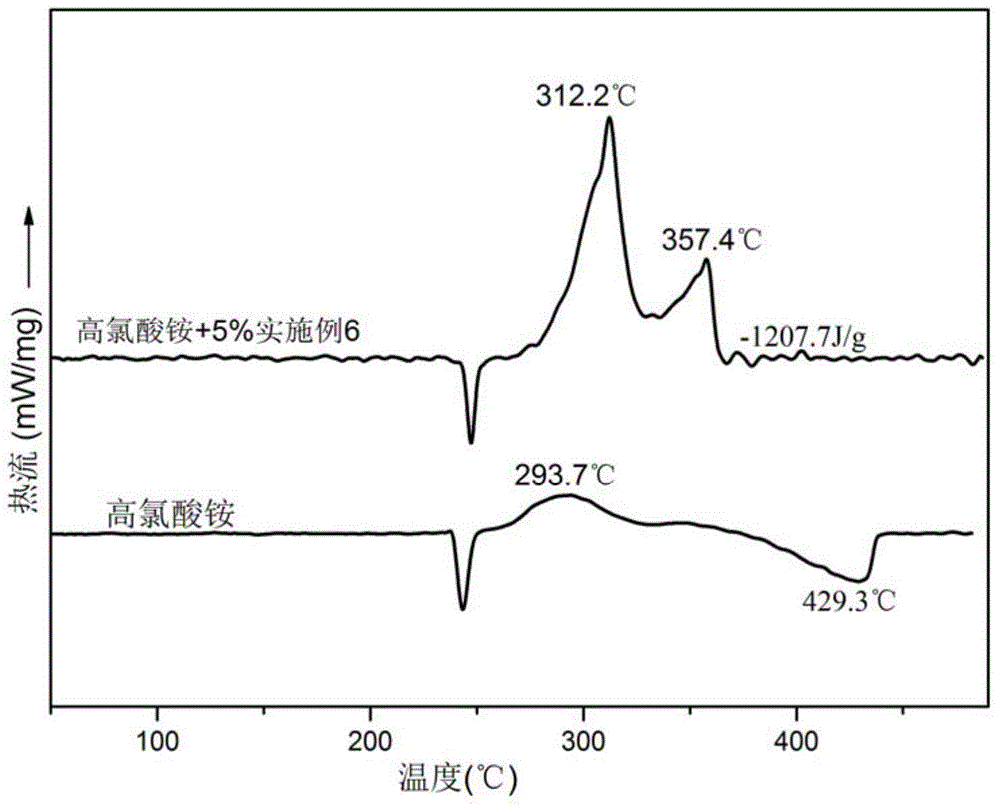
Ferrocene tetrazole ionic compounds and preparation method thereof
A technology for ferrocene tetrazolium and ionic compounds, which is applied in the field of ferrocene tetrazolium ionic compounds and their preparation, can solve the problem that the catalytic effect is not very good, the iron content of the catalyst is low, and the mechanical properties and technological properties cannot be obtained. and combustion performance, etc., to achieve the effects of low synthesis cost, good thermal stability and non-volatile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of ferrocene tetrazolium ion compound with the following structural formula
[0033]
[0034] Completely dissolve 0.232g (0.91mmol) of compound A in a 50mL round-bottomed flask filled with 10mL of absolute ethanol. After the solution is completely transparent, add 0.133g (0.76mmol) of 1-methanol dissolved in 10mL of absolute ethanol dropwise. -3-butylimidazolium chloride salt solution, a white precipitate was formed immediately, stirred at room temperature for 6 hours, filtered with suction, the filtrate was spin-dried, dissolved in dichloromethane, filtered, spin-dried, and recrystallized with acetone / ether. Then the filter cake was dried in a vacuum oven at 50° C. for 24 hours to obtain 0.237 g of a ferrocene tetrazolium ion compound with a melting point of 125.2° C. and a yield of 79%.
[0035] The spectral data of the resulting product is: 1 H NMR (400MHz, DMSO) δ (ppm): 9.14 (s, 1H), 7.74 (d, J = 27.4Hz, 2H), 4.68 (t, J = 1.8Hz, 2H), 4.19 (dd, J = 9...
Embodiment 2
[0037] Preparation of ferrocene tetrazolium ion compound with the following structural formula
[0038]
[0039] In Example 1, the 1-methyl-3-butylimidazolium chloride salt used is replaced by equimolar 1-methyl-3-octylimidazolium chloride salt, and other steps are the same as in Example 1 to obtain ferrocene Tetrazolium ion compound 0.252g, its melting point is 99.9°C, and the yield is 74%.
[0040] The spectral data of the resulting product is: 1 H NMR (400MHz, DMSO) δ (ppm): 9.12 (s, 1H), 7.75 (d, J = 18.2Hz, 2H), 4.68 (t, J = 1.8Hz, 2H), 4.34-4.04 (m, 4H ), 3.90(d, J=43.1Hz, 7H), 1.77(s, 2H), 1.25(s, 10H), 0.85(d, J=6.6Hz, 3H); 13 C NMR (400MHz, DMSO) δ (ppm): 158.86, 136.86, 123.72, 122.34, 99.50, 78.04, 68.79, 67.48, 66.66, 48.83, 35.94, 31.14, 29.40, 28.46, 28.32, 25.49, 22.033, 13. (theoretical calculation values in brackets): C% 61.60 (61.61), H% 7.12 (7.19), N% 18.65 (18.74).
Embodiment 3
[0042] Preparation of ferrocene tetrazolium ion compound with the following structural formula
[0043]
[0044] In Example 1, the 1-methyl-3-butylimidazolium chloride salt used is replaced by equimolar 1-methyl-3-dodecylimidazolium chloride salt, and other steps are the same as in Example 1 to obtain two 0.299 g of ferrocene tetrazolium ion compound, its melting point is 83.4°C, and the yield is 78%.
[0045] The spectral data of the resulting product is: 1 H NMR (400MHz, DMSO) δ (ppm): 9.18 (s, 1H), 7.77 (s, 2H), 4.67 (s, 2H), 4.19 (s, 4H), 3.90 (d, J = 38.2Hz, 7H ), 1.76(s, 2H), 1.24(s, 17H), 0.85(s, 3H); 13 C NMR (400MHz, DMSO) δ (ppm): 158.91, 123.70, 122.32, 78.44, 68.76, 67.36, 66.63, 48.84, 31.26, 29.39, 28.98, 28.92, 28.80, 28.67, 28.36, 25.49, 22.06, 13.9 (theoretical calculation values in brackets): C% 64.20 (64.28), H% 7.91 (7.99), N% 16.59 (16.66).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Heat release | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com