Microbial limit detection method for membrane used for multi-layer coextrusion infusion
A technology of microbial limit and infusion membrane, which is applied in the detection field of microbial limit, can solve problems such as secondary pollution, achieve the effects of reducing pollution, accurate and reliable experimental results, and avoiding secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
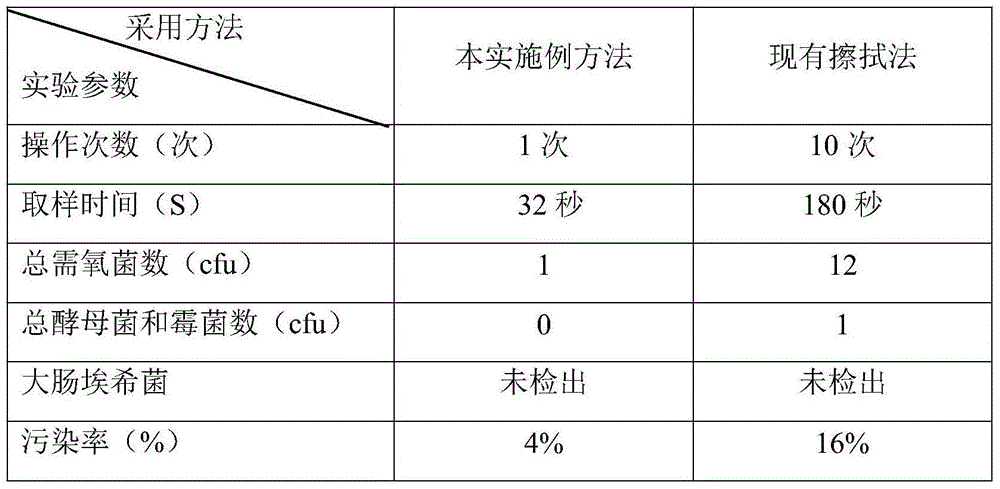
[0030] A method for detecting the microbial limit of a multilayer co-extruded transfusion film, the specific detection steps of which are as follows:
[0031] (1) Under a sterile environment, take the multi-layer co-extrusion infusion membrane, wash the outer layer of the multi-layer co-extrusion infusion membrane with sterile saline at least 3 times, and take a 50cm 2 Membrane material, equivalent to a single layer area of 100cm 2 ,.
[0032] (2) Take the 50cm 2 Cut the membrane material into pieces with sterile scissors, cut into small pieces of 5cm×0.5cm, put them in a sterile Erlenmeyer flask, add 100ml of sterile sodium chloride-peptone buffer solution with a pH of 7.0, shake for 1 minute, and let stand 10 minutes to prepare the test solution.
[0033] (3) In order to make the filter membrane smooth, first wet the filter membrane of the membrane filter with 10ml of sterile sodium chloride peptone buffer solution with a pH of 7.0, then take 10ml of the test solution t...
Embodiment 2
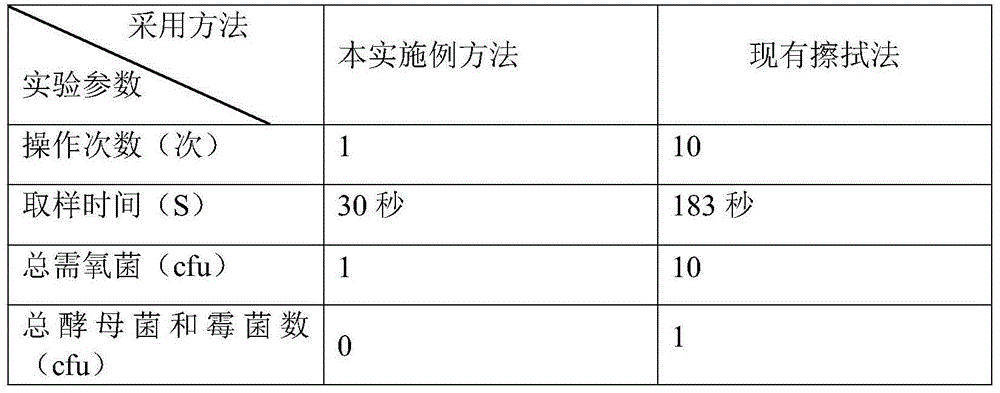
[0045] A method for detecting the microbial limit of a multilayer co-extruded transfusion film, the specific detection steps of which are as follows:
[0046] (1) Under a sterile environment, take the multi-layer co-extrusion infusion membrane, wash the outer layer of the multi-layer co-extrusion infusion membrane with sterile saline at least 3 times, and take a 50cm 2 Membrane material, equivalent to a single layer area of 100cm 2 . .
[0047] (2) Take the 50cm 2 Cut the membrane material into pieces with sterile scissors, cut into small pieces of 5cm×0.5cm, put them in a sterile Erlenmeyer flask, add 100ml of sterile sodium chloride-peptone buffer solution with a pH of 7.0, shake for 1 minute, and let stand 10 minutes to make the test solution.
[0048] (3) In order to make the filter membrane smooth, first wet the filter membrane of the membrane filter with 10ml of sterile sodium chloride peptone buffer solution with a pH of 7.0, then take 10ml of the test solution an...
Embodiment 3
[0061] A method for detecting the microbial limit of a multilayer co-extruded transfusion film, the specific detection steps of which are as follows:
[0062] (1) Under a sterile environment, take the multi-layer co-extrusion infusion membrane, wash the outer layer of the multi-layer co-extrusion infusion membrane with sterile saline at least 3 times, and take a 50cm 2 Membrane material, equivalent to a single layer area of 100cm 2 .
[0063] (2) Take the 50cm 2 Cut the membrane material into pieces with sterile scissors, cut into small pieces of 5cm×0.5cm, put them in a sterile Erlenmeyer flask, add 100ml of sterile sodium chloride-peptone buffer solution with a pH of 7.0, shake for 1 minute, and let stand 10 minutes to make the test solution.
[0064] (3) In order to make the filter membrane smooth, first wet the filter membrane of the membrane filter with 10ml of sterile sodium chloride peptone buffer solution with a pH of 7.0, then take 10ml of the test solution and f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com