Preparation method of polyacrylic acid hollow microgel
A technology of polyacrylic acid and microgel, which is applied in the field of preparation of polyacrylic acid hollow microgel to achieve good emulsification stability, easy implementation, and good monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
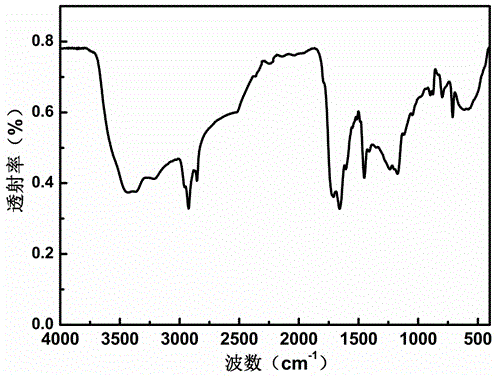
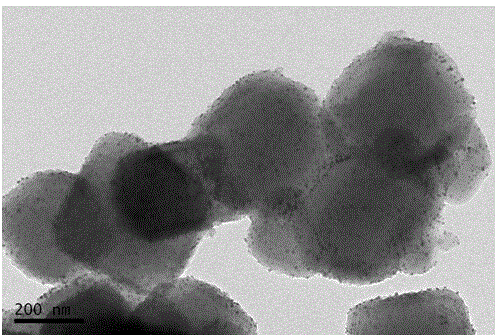
Image
Examples
Embodiment 1
[0036] 12g of acrylonitrile, 1.2g of divinylbenzene, 0.8g of hexadecane, 2.4g of isooctane, 10g of ethyl orthosilicate, 1.0g of γ-(methacryloyloxy)propyltrimethoxysilane and 0.2 g azobisisoheptanonitrile was magnetically stirred for 30 minutes to fully dissolve it as an oil phase;
[0037]Weigh 0.02g of sodium nitrite and dissolve it in deionized water with magnetic stirring for 10min to fully dissolve it as the water phase; slowly add the oil phase into the water phase, then add 1g of triethylamine, stir magnetically at room temperature for 30min to form a mixed solution; Then use a high-speed shearing device to homogeneously emulsify for 5 minutes at a shear rate of 16000 rpm under an ice-water bath to form a fine emulsion;
[0038] Transfer the fine emulsion to a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser, pass nitrogen gas for 30 minutes under stirring to remove the air in the system, and react at a constant temperature in a water bath...
Embodiment 2
[0040] 12g of acrylonitrile, 1.2g of divinylbenzene, 0.8g of hexadecane, 1.2g of isooctane, 10g of ethyl orthosilicate, 1.0g of γ-(methacryloyloxy)propyltrimethoxysilane and 0.2 g azobisisoheptanonitrile was magnetically stirred for 30 minutes to fully dissolve it as an oil phase;
[0041] Weigh 0.02g of sodium nitrite and dissolve it in deionized water and stir it magnetically for 10 minutes to make it fully dissolved as the water phase;
[0042] Slowly add the oil phase to the water phase, then add 1g of triethylamine, stir magnetically at room temperature for 30 minutes to form a mixed solution; then use a high-speed shearing device in an ice-water bath to homogeneously emulsify at a shear rate of 16000rpm for 5 minutes to form a fine emulsion ;
[0043] Transfer the fine emulsion to a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser, pass nitrogen gas for 30 minutes under stirring to remove the air in the system, and react at a constant tem...
Embodiment 3
[0045] 12g of acrylonitrile, 1.2g of divinylbenzene, 0.8g of hexadecane, 2.4g of isooctane, 10g of ethyl orthosilicate, 2.0g of γ-(2,3-epoxypropoxy)propyltrimethoxy Silane and 0.2 g of azobisisoheptanonitrile were magnetically stirred for 30 minutes to fully dissolve it as an oil phase;
[0046] Weigh 0.02g of sodium nitrite and dissolve it in deionized water with magnetic stirring for 10min to fully dissolve it as the water phase; slowly add the oil phase into the water phase, then add 1g of triethylamine, stir magnetically at room temperature for 30min to form a mixed solution; Then use a high-speed shearing device to homogeneously emulsify for 5 minutes at a shear rate of 16000 rpm under an ice-water bath to form a fine emulsion;
[0047] Transfer the fine emulsion to a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser, pass nitrogen gas for 30 minutes under stirring to remove the air in the system, and react at a constant temperature in a wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com