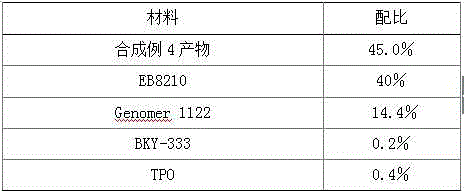
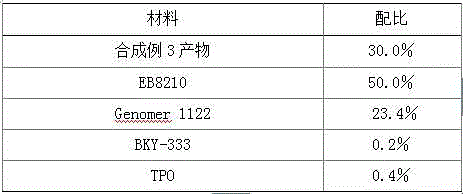
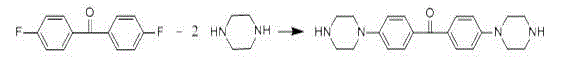Application of low-viscosity light-cured resin in 3D (three-dimensional) printing materials
A light-curing resin and 3D printing technology, applied in the field of 3D printing, can solve the problems of dependence on foreign imports of molding resins, few suitable materials for light-curing, and obstacles to rapid development, etc., to reduce amine mobility, no catalyst residue, and curing shrinkage Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0067] Synthesis Example 1 Polyether Acrylate 1
[0068] A kind of polyether acrylate and preparation method thereof, comprises following two steps:
[0069] a. Transesterification reaction: Take 400g polyether triol (Mn≈700, Dow VOANOL 2070), 343.2g ethyl acrylate, 1.2g trinonylphenyl phosphite, 0.2g 2,6-di-tert-butyl Base-4-methylphenol, after uniform dispersion, add 5g of immobilized enzyme Novozyme 435 and 0.1g of lanthanum dodecylsulfonate, stir and react at 40°C for 24h;
[0070] b. Purification treatment: After filtering the mixture obtained in the previous step, the filtrate was subjected to vacuum rotary evaporation, and the ethanol and excess methacrylic acid generated by the reaction were removed to obtain 490.2 g of transparent and colorless esterified product (solid content 99.2%, viscosity 85 mPa ·s / 25°C, the esterification rate (based on polyether polyol as the base substrate, the same below) is 97.4%), the molecular weight of the obtained product is 862,...
Synthetic example 2
[0071] Synthesis Example 2 Branched Polyether Acrylate 2 (Mn≈1022, Functionality 9)
[0072] A kind of branched polyether acrylate and preparation method thereof, concrete steps are as follows:
[0073] (1) Synthesis of branched polyether polyols
[0074] Glycerol is used as raw material to form branched polyether polyols (Mn≈536, functionality 9, branching degree 0.72) through one-step condensation under acidic conditions;
[0075] (2) Enzyme-catalyzed transesterification reaction, including the following two steps:
[0076] a. Transesterification reaction: take 300g of the above-mentioned branched polyether polyol, 550.0g methyl acrylate, 1.8g trinonylphenyl phosphite, 0.25g p-hydroxyanisole, after dispersing evenly, add 8g of curing enzyme Novozyme 435 and 0.1g lanthanum dodecylsulfonate, stirred and reacted at 50°C for 24h; the chemical reaction formula is as follows:
[0077]
[0078] b. Purification treatment: After filtering the mixture obtained in the previous ...
Synthetic example 3
[0079] Synthesis example 3 polycyclic amine modified branched polyether acrylate 3
[0080] (1) Synthesis of heteropolycyclic amines
[0081] Add 218.2g of 4,4'-difluorobenzophenone, 175.0g of anhydrous piperazine, 150.0g of anhydrous potassium carbonate and 300ml of dimethyl sulfoxide to a four-necked flask equipped with a condenser tube, a thermometer and a stirrer ; Under the protection of nitrogen, heat up to 100 ° C for 12 hours; when it is cooled to 80 ° C, filter while it is hot, wash the filtrate twice with water, add anhydrous sodium sulfate to dry, filter again, and distill off the solvent under reduced pressure to obtain Heteropolycyclic semi-finished product 345.0g, yield 87.7%; Reaction equation is as follows:
[0082]
[0083] (2) Preparation of polycyclic amine modified polyether acrylate 1
[0084] Take 35.1g (0.1mol) of the heteropolycyclic amine prepared in step (1) and put it into a three-necked flask, add 0.6g of DBU catalyst, and gradually raise the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Functional group degree | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com