Medicinal ranitidine hydrochloride composition capsule for treating gastric ulcer
A technology of ranitidine hydrochloride and a composition, which is applied in the field of drug ranitidine hydrochloride composition capsules for treating gastric ulcer, can solve the problems of poor stability, easy oxidation of products, difficult filling, etc., and achieves the improvement of stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
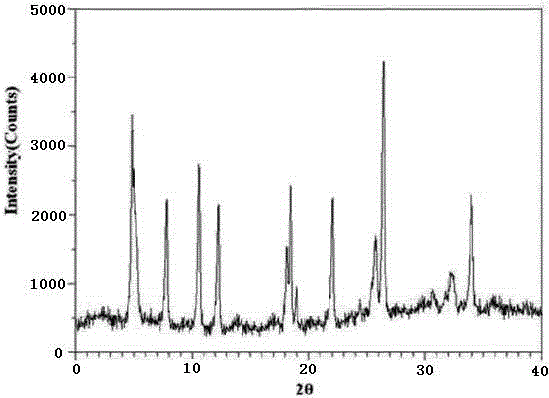
[0030] Example 1: Preparation of ranitidine hydrochloride crystals
[0031] (1) Prepare methanol and N,N-dimethylformamide as a mixed solvent, and the volume ratio of methanol and N,N-dimethylformamide is 2.5:1;
[0032] (2) Take the ranitidine hydrochloride raw material, dissolve it in the mixed solvent of methanol and N,N-dimethylformamide whose volume is 8 times the weight of ranitidine hydrochloride in step (1), and stir until all Dissolving to obtain ranitidine hydrochloride solution;
[0033] (3) At a temperature of 8°C and a stirring speed of 240r / min, add the ranitidine hydrochloride solution in step (2) into 80% ethanol whose volume is 10 times the weight of ranitidine hydrochloride, and mix to form Cool the suspension to -5°C at a rate of 15°C / min;
[0034] (4) Carry out suction filtration, wash the filter cake with 95% ethanol, and then vacuum-dry the filter cake at 35°C to obtain a crystalline powder, which is the ranitidine hydrochloride compound.
[0035] Th...
Embodiment 2
[0036] Example 2: Preparation of Ranitidine Hydrochloride Capsules:
[0037] Prescription: in parts by weight as shown in Table 1
[0038] Table 1 The composition prescription of ranitidine hydrochloride
[0039]
[0040] 1) Processing of raw and auxiliary materials: pass ranitidine hydrochloride and anhydrous lactose through a 60-mesh sieve with a vibrating sieving machine;
[0041] 2) Weighing: Weigh the raw and auxiliary materials according to the prescription;
[0042] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the motor operating frequency to 200r / min, and start the mixer to mix for 50 minutes;
[0043] 4) Filling by automatic capsule filling machine, the difference in filling volume must meet the national standard;
[0044] 5) Packaging.
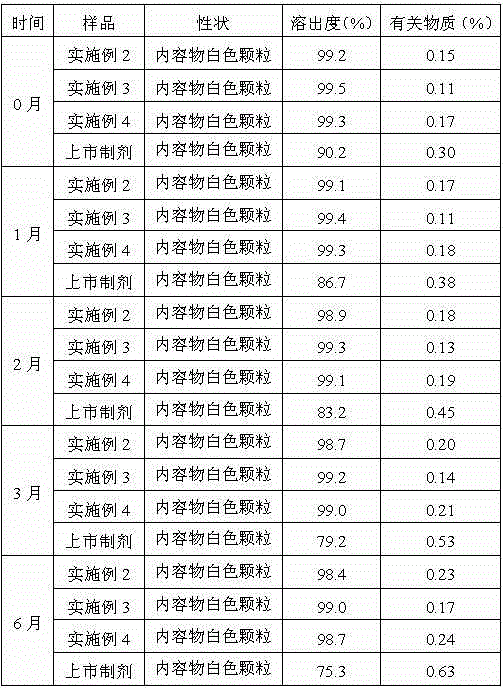
Embodiment 3
[0045] Example 3: Preparation of Ranitidine Hydrochloride Capsules:
[0046] Prescription: in parts by weight as shown in Table 2
[0047] Table 2 Prescription of ranitidine hydrochloride composition
[0048]
[0049] 1) Processing of raw and auxiliary materials: pass ranitidine hydrochloride and anhydrous lactose through a 60-mesh sieve with a vibrating sieving machine;
[0050] 2) Weighing: Weigh the raw and auxiliary materials according to the prescription;
[0051] 3) Mixing: Add the weighed raw and auxiliary materials into the mixer, set the motor operating frequency to 200r / min, and start the mixer to mix for 50 minutes;
[0052] 4) Filling by automatic capsule filling machine, the difference in filling volume must meet the national standard;
[0053] 5) Packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com