Injectable-porous-drug loaded polymethyl methacrylate-based composite scaffold bone transplant material and preparation method thereof
A technology of polymethyl methacrylate and composite scaffolds, applied in medical science, prostheses, etc., can solve the problems of mismatching mechanical properties with natural bone, high polymerization temperature, and shedding of surrounding tissues, so as to reduce the cost of treatment , mechanical characteristics matching, and the effect of improving the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
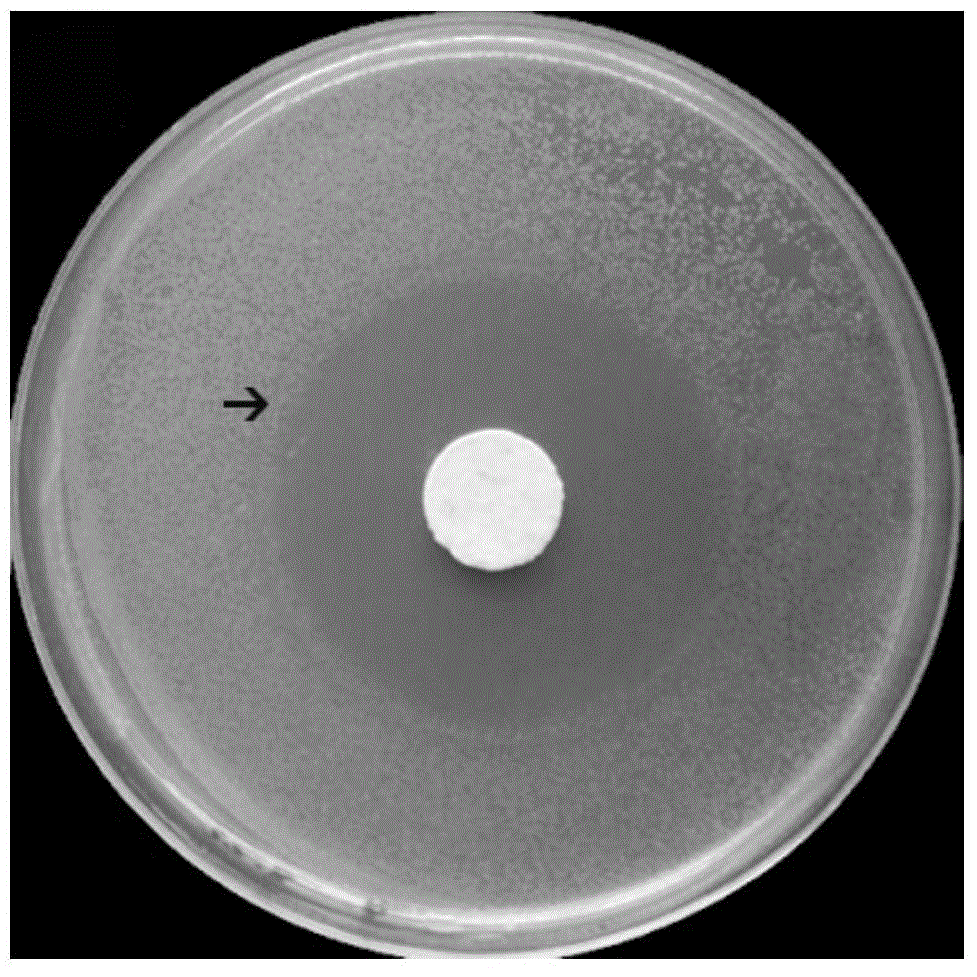
Examples
Embodiment 1
[0042] (1) Dissolve chitosan (CS) in 0.1mol / L hydrochloric acid solution at room temperature to prepare CS hydrochloric acid solution with a mass concentration of 2%. After the dissolution is complete, stir in an ice bath for 15 minutes.
[0043] (2) At the same time, dissolve sodium glycerophosphate (GP) in distilled water to prepare a GP solution with a mass concentration of 50%, and also place it in an ice bath for 15 minutes.
[0044] (3) Under continuous stirring, slowly drop 0.5mL GP solution into 4.5mL CS hydrochloric acid solution, keep the solution without turbidity, and stir for 20min to obtain a well-mixed CS-GP solution.
[0045] (4) Add 25mg of gentamicin sulfate (GM) and 300mg of hydroxyapatite (HA) to the well-mixed CS-GP solution, and ultrasonically treat it to make it evenly mixed to obtain CS-GP / Nano-HA / GM mixture.
[0046] (5) Put the CS-GP / Nano-HA / GM mixed solution in a 37°C incubator, and form a CS-GP / Nano-HA / GM gel after 5 minutes.
[0047] (6) CS-GP / Na...
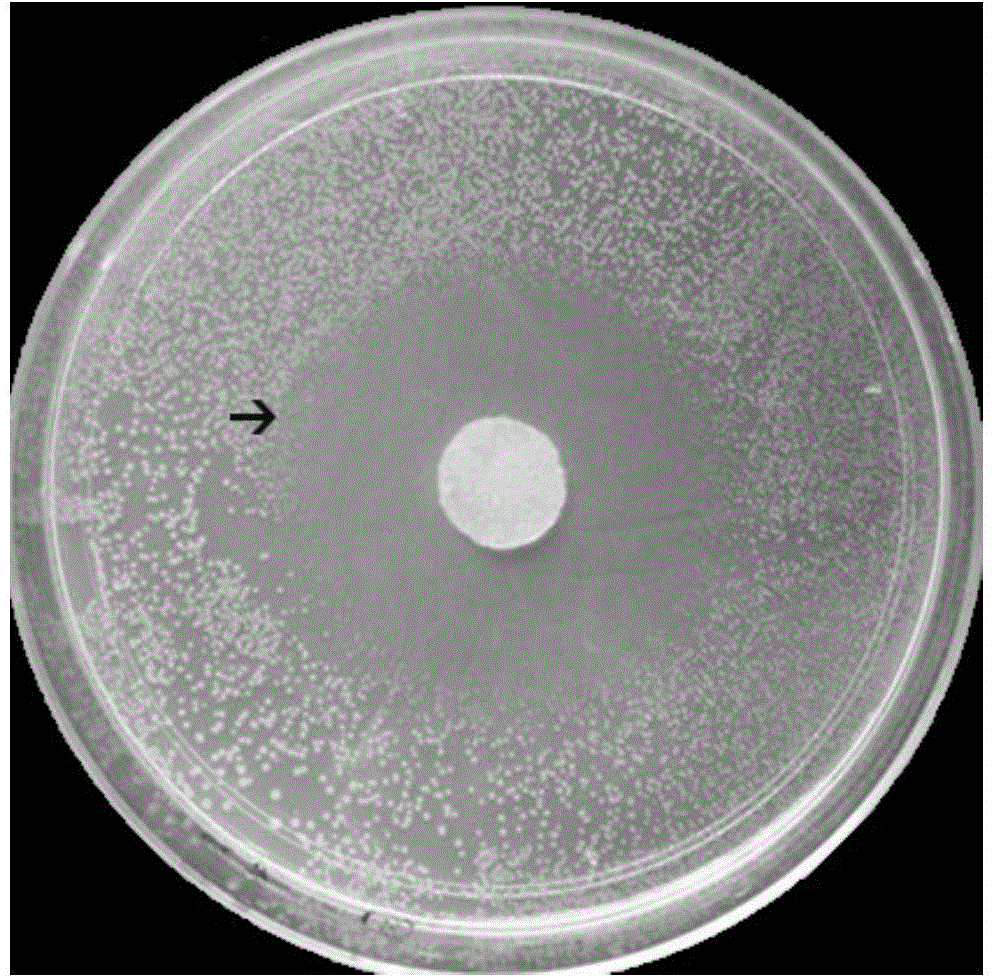
Embodiment 2
[0050] (1) Dissolve chitosan (CS) in 0.1mol / L hydrochloric acid solution at room temperature to prepare CS hydrochloric acid solution with a mass concentration of 2%. After the dissolution is complete, stir in an ice bath for 15 minutes.
[0051] (2) At the same time, dissolve sodium glycerophosphate (GP) in distilled water to prepare a GP solution with a mass concentration of 50%, and also place it in an ice bath for 15 minutes.
[0052] (3) Under continuous stirring, slowly drop 0.5mL GP solution into 4.5mL CS hydrochloric acid solution, keep the solution without turbidity, and stir for 20min to obtain a well-mixed CS-GP solution.
[0053] (4) Add 25mg of vancomycin (VM) and 300mg of hydroxyapatite (HA) to the well-mixed CS-GP solution, and ultrasonically treat it to make it evenly mixed to obtain a CS-GP / Nano-HA / VM mixed solution .
[0054] (5) Put the CS-GP / Nano-HA / VM mixed solution in a 37°C incubator, and form a gel after 5 minutes.
[0055] (6) CS-GP / Nano-HA / VM gel wa...
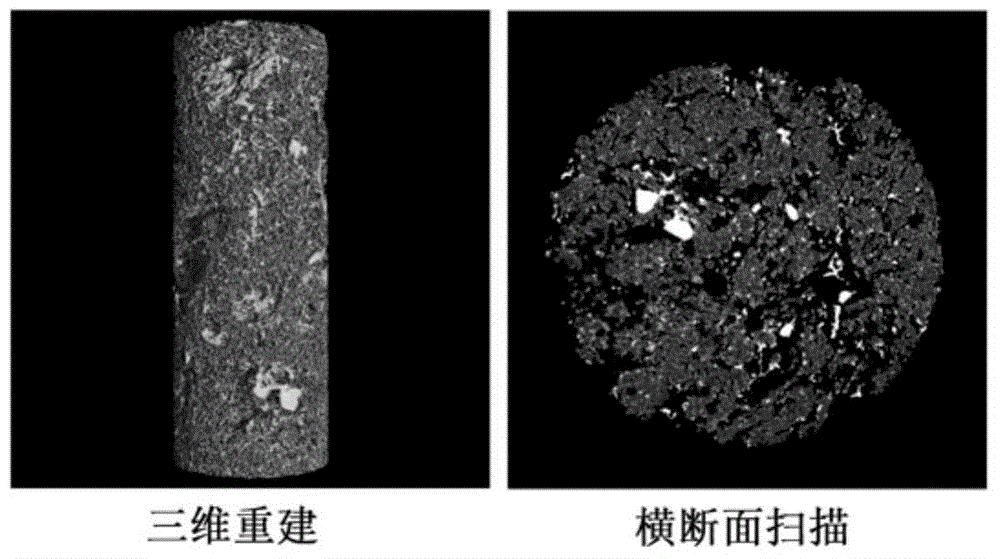
Embodiment 3
[0058] (1) Dissolve chitosan (CS) in 0.1mol / L hydrochloric acid solution at room temperature to prepare CS hydrochloric acid solution with a mass concentration of 2%. After the dissolution is complete, stir in an ice bath for 15 minutes.
[0059] (2) At the same time, dissolve sodium glycerophosphate (GP) in distilled water to prepare a GP solution with a mass concentration of 50%, and also place it in an ice bath for 15 minutes.
[0060] (3) Under continuous stirring, slowly drop 0.5mL GP solution into 4.5mL CS hydrochloric acid solution, keep the solution without turbidity, and stir for 20min to obtain a well-mixed CS-GP solution.
[0061] (4) Add 25mg of gentamicin sulfate (GM) and 300mg of hydroxyapatite (HA) to the well-mixed CS-GP solution, and ultrasonically treat it to make it evenly mixed to obtain CS-GP / Nano-HA / GM mixture.
[0062] (5) Put the CS-GP / Nano-HA / GM mixed solution in a 37°C incubator, and form a CS-GP / Nano-HA / GM gel after 5 minutes.
[0063] (6) Stir and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com