Preparation method and application of 9,9'-(4,4'-biphenyl)bisfluorone bromination reagent
A technology of bifluorone and biphenyl, which is applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials, can solve the problems of unsatisfactory sensitivity and selectivity, and achieve improved sensitivity, fast response, and simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
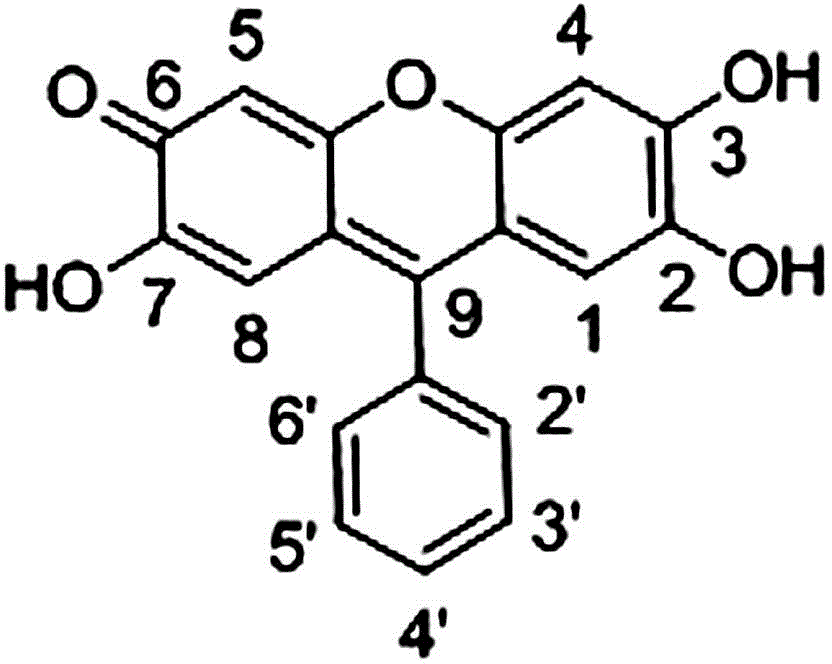
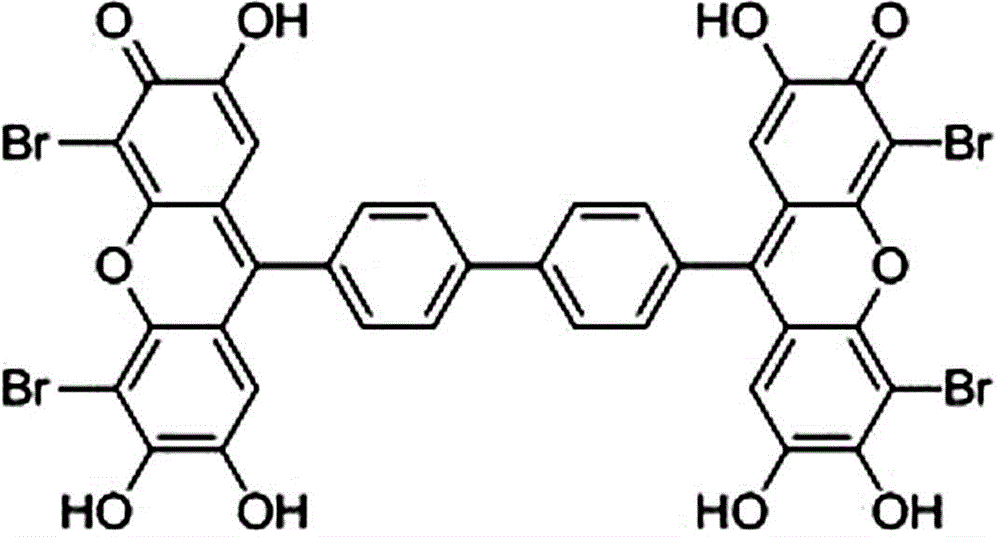
[0030] Such as figure 2 As shown, a 9,9'-(4,4'-biphenyl)bisfluorone brominated reagent, referred to as 9,9'-QBBPBF, has a molecular structure of
[0031] .
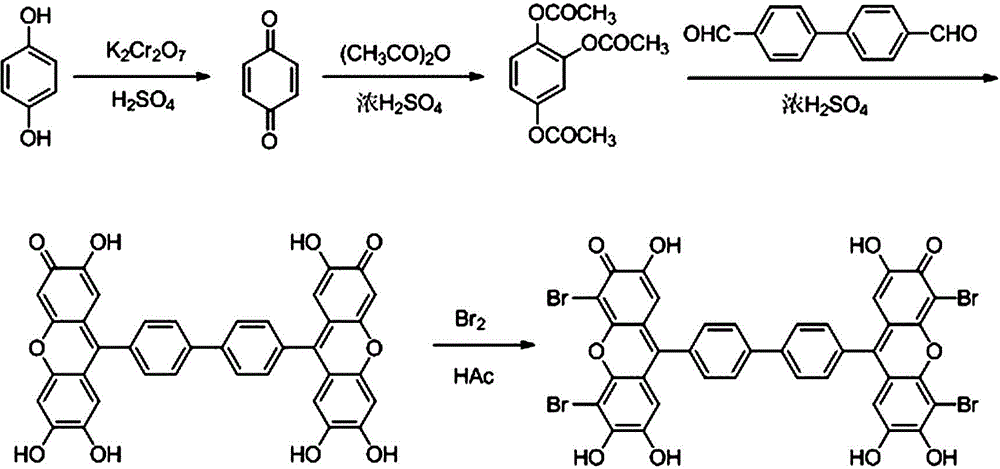
[0032] Such as image 3 Shown, the preparation method of 9,9'-QBBPBF comprises the following steps:
[0033] (1) Synthesis of p-benzoquinone: Dissolve 60g of hydroquinone in 1000mL of water at 50°C, cool to a temperature of 20°C, and slowly add 32mL of concentrated H 2 SO 4 , stir vigorously, control the reaction temperature ≤ 30°C, add 400mL of K with a concentration of 0.2g / ml dropwise at a rate of 0.5~1.0mL / min 2 Cr 2 o 4 The solution was cooled to room temperature after the dropwise addition, filtered with suction, and the precipitate was washed with deionized water until the filtrate was light yellow, and the precipitate was dried at 80°C to obtain 32.3 g of yellow p-benzoquinone with a yield of 54%;
[0034] (2) Synthesis of trimerol triacetate: In a 100mL beaker, add 28.0mL acetic anhydride and 1.0mL conc...
experiment example 1
[0037] Experimental example 1: Photometric analysis and detection of Mo(VI) in alkaline medium.
[0038] In a 25mL volumetric flask, add 5μg Mo(VI) standard solution, 2.0% Triton X-100 aqueous solution 2.0mL, 0.3g / L 9,9'-QBBPBF ethanol solution 3.0mL, pH8.0 borax- Sodium hydroxide buffer solution 1.5mL, dilute to the mark with water, shake well, take the blank reagent as a reference, measure the absorbance of the complex at 670nm with a 1cm cuvette, the apparent molar absorptivity is 1.9×10 5 L·mol -1 cm -1 . The comparison results of the sensitivity of Mo(VI) measured by the photometric analysis method of the present invention and the sensitivity of molybdenum measured by existing similar reagents are shown in Table 1, and the results show that the photometric method of the present invention for molybdenum ions has high sensitivity.
[0039] Table 1: Sensitivity of several reagents for determination of molybdenum
[0040]
[0041] The comparison results of the sensitiv...
experiment example 2
[0047] Experimental Example 2: Fluorescent detection of Mn(II) by 9,9'-QBBPBF.
[0048] Sample processing: According to the method in the literature [Du Rongshan, Huang Liping, Journal of Analytical Science, 2010, 26(5), 535-538], take two different urine samples of 100 mL each, and place them in Erlenmeyer flasks respectively. Add 25.0mL concentrated nitric acid, 4.0mL perchloric acid, heat and digest until colorless, and concentrate to 1.0-2.0mL. Take it off to cool, add a small amount of water, transfer to a 10mL centrifuge tube, and wash the Erlenmeyer flask twice with a small amount of twice distilled water, and pour the washing solution into the centrifuge tube. Centrifuge at 8000r / min for 10min, then transfer all the supernatant into a beaker, and use 0.2mol / L NH 3 ·H 2 O to neutralize to PH=7.0, finally dilute to 100mL volumetric flask, shake well. Take 5.00mL of the test solution, add 1.00mL167μg / mL ascorbic acid solution, the Fe in the sample 3+ reduced to Fe 2+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com