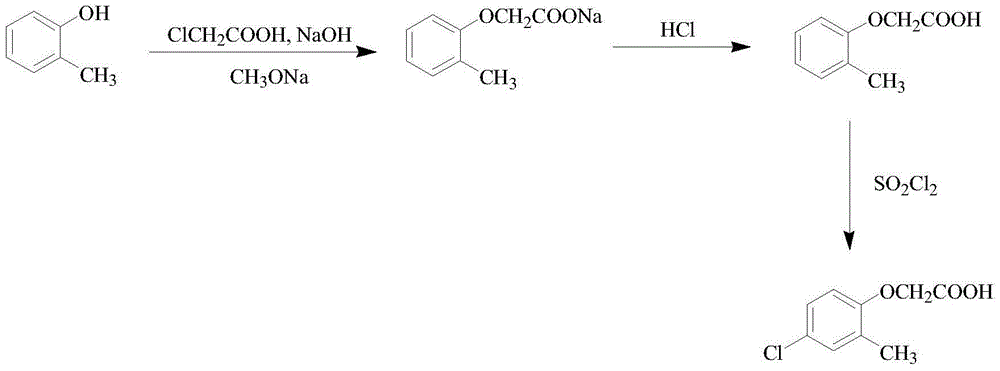
Synthetic method of 2-methyl-4-chlorophenoxyacetic acid
A technology of o-methylphenoxyacetic acid and chlorophenoxyacetic acid is applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc. phenol wastewater, incomplete neutralization and other problems, to achieve the effect of reducing energy consumption, improving production economy, and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
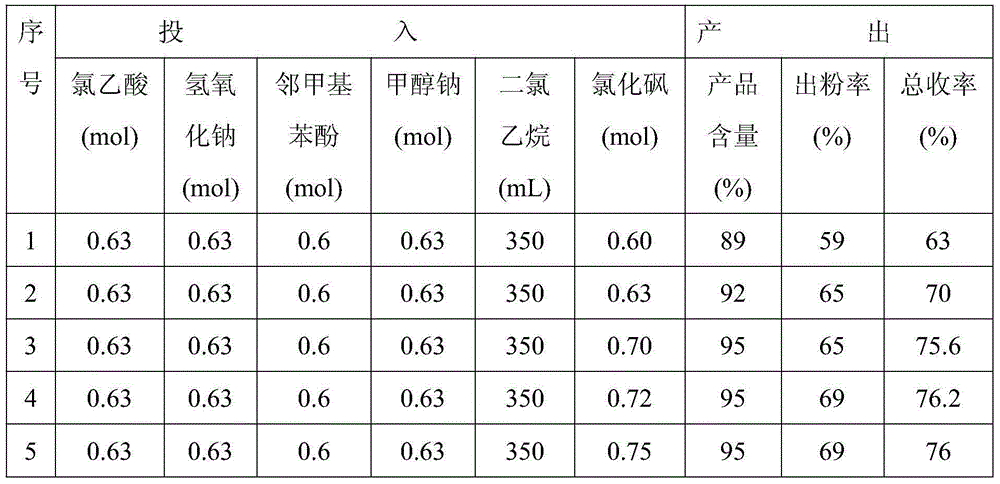
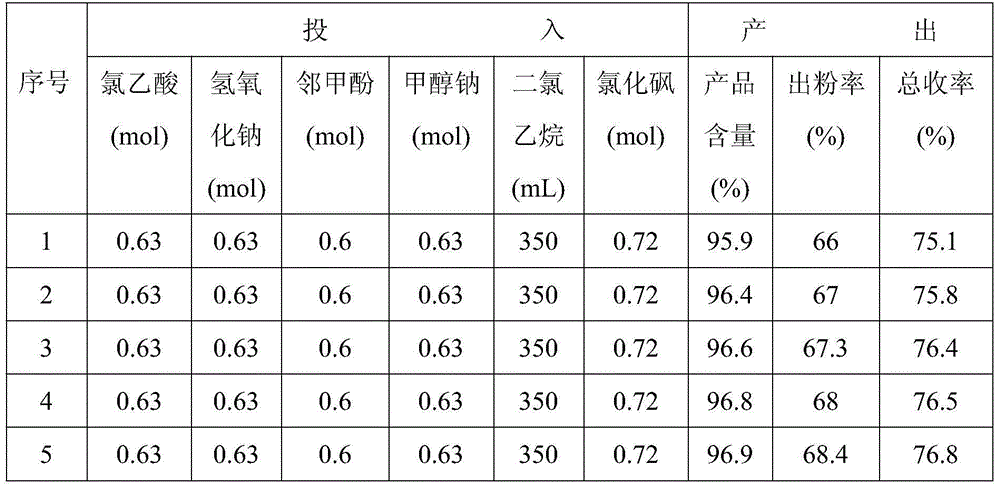
Examples
Embodiment 1
[0022] A kind of synthetic method of 2-methyl-4-chlorophenoxyacetic acid, comprises the following steps:
[0023] (1) Preparation of sodium methoxide solution: first add methanol to the reaction flask, then add sliced sodium metal under stirring, react at room temperature for 30 minutes, then heat up to reflux state and keep warm for 30 minutes to obtain sodium methoxide solution;
[0024] (2) Preparation of sodium chloroacetate solution: under stirring, chloroacetic acid is first dissolved in water, after chloroacetic acid is completely dissolved, sodium hydroxide solution is added dropwise at low temperature, when pH=8 is the reaction end point, retest pH=10 minutes later At 8 o'clock, it shows that sodium chloroacetate has been prepared;
[0025] (3) Preparation of o-methylphenoxyacetic acid: add o-methylphenol and sodium methoxide solution to the reaction flask, heat up to reflux under stirring, keep warm for a period of time to remove methanol, remove until the material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com