Method for synthesizing 1,2-propylene glycol using glucose
A technology of propylene glycol and glucose, which is applied in the field of glucose hydrogenolysis to prepare polyols, to avoid corrosion, solve equipment corrosion, and operate easily
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The hydrogenolysis catalyst is a supported Cu, Ni, CuNi metal catalyst, the carrier is MgO, ZnO, and the specific surface area of the catalyst is 200-300m 2 / g, the pore volume is 0.4-0.7cm 3 / g, the average pore size distribution is 3-6nm. The metal precursors are respectively nickel nitrate, copper nitrate, magnesium nitrate or zinc nitrate. The supported metal catalyst is prepared by a co-precipitation method through the steps of precipitation-drying-roasting and the like. The total molar loading of metal Cu and Ni is 30%, and the metal loading of Mg / Zn is 70%.
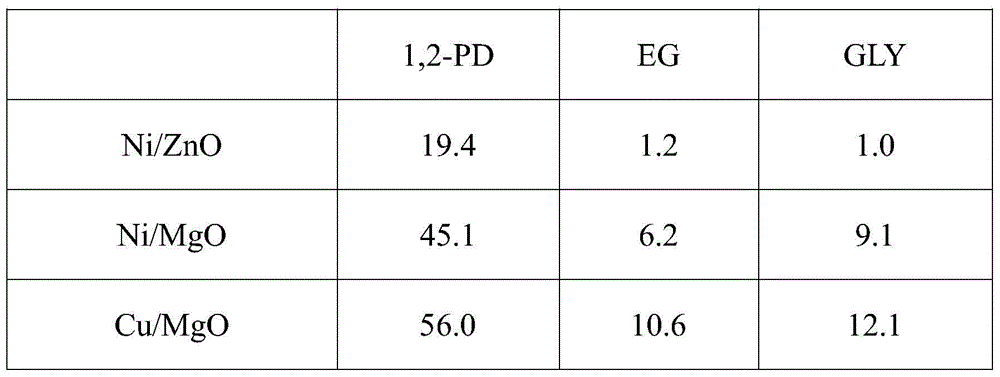
[0019] Glucose reaction results on different catalysts in the following table 1
[0020]
[0021]
[0022] Reaction conditions: 10wt% glucose aqueous solution 30g, catalyst 0.3g, Ca(OH) 2 0.08g, reaction pressure 6MPa, reaction temperature 140°C, reaction 2h, temperature raised to 220°C, reaction 3h. (EG in the table is ethylene glycol; 1,2-PD is 1,2-propanediol; GLY is glycerin)
[0023] It ca...
Embodiment 2
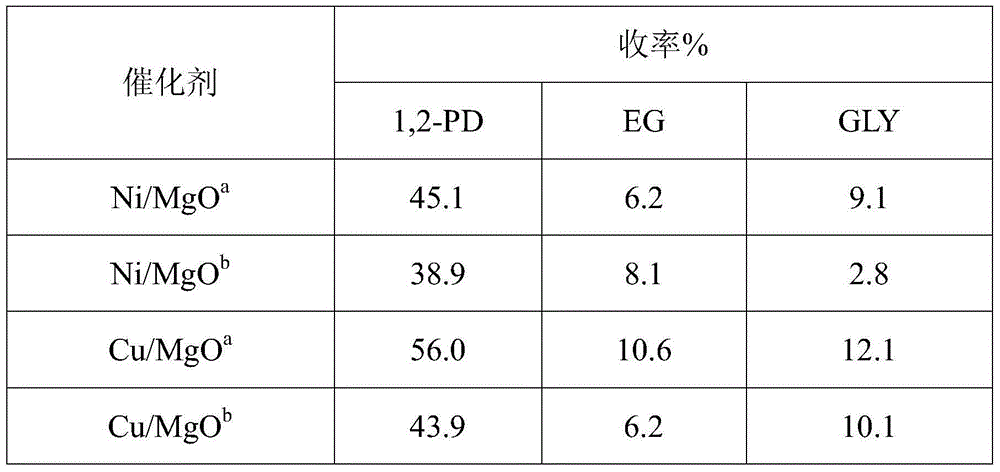
[0025] Based on the basicity of MgO, the basicity of the support in the Cu / MgO and Ni / MgO catalysts was respectively investigated instead of the solid base Ca(OH) 2 Effect on C-C Fragmentation. The following table 2 sees the impact of not adding solid base on the reaction result.
[0026]
[0027] a Ca(OH) 2 0.08g; b No Ca(OH) added 2 . Other reaction conditions: 10wt% glucose aqueous solution 30g, catalyst 0.3g, reaction pressure 6MPa, reaction temperature 140°C, reaction for 2h, temperature raised to 220°C, reaction for 3h. (EG in the table is ethylene glycol; 1,2-PD is 1,2-propanediol; GLY is glycerin)
[0028] It can be seen from Table 2 that in the low-concentration glucose hydrogenolysis reaction, the yield of 1,2-propanediol is slightly reduced, and the alkalinity of MgO can replace part of the solid base Ca(OH) 2 It plays a role in promoting C-C cleavage, and improves the yield of 1,2-propanediol by changing the reaction conditions.
Embodiment 3
[0030] Based on the fact that Cu / MgO and Ni / MgO catalysts have high selectivity in catalyzing the hydrogenolysis of glucose, the influence of CuNi / MgO with different metal ratios on the hydrogenolysis of glucose was considered. Table 3 below shows the reaction results of CuNi / MgO catalyzed hydrogenolysis of glucose with different metal ratios.
[0031]
[0032] Reaction conditions: 30g of 10wt% glucose aqueous solution, 0.3g of catalyst, no addition of Ca(OH) 2 , Reaction pressure 6MPa, reaction temperature 140°C, react for 2h, heat up to 220°C, react for 3h. (EG in the table is ethylene glycol; 1,2-PD is 1,2-propanediol; GLY is glycerol)
[0033] As can be seen from Table 3, with respect to other catalysts, the catalytic activity of 1Ni4Cu / MgO catalyst is relatively high, and the yield of 1,2-propanediol reaches 46.7%. Preliminary results show that 1Ni4Cu / MgO catalyst has certain potential for glucose conversion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com