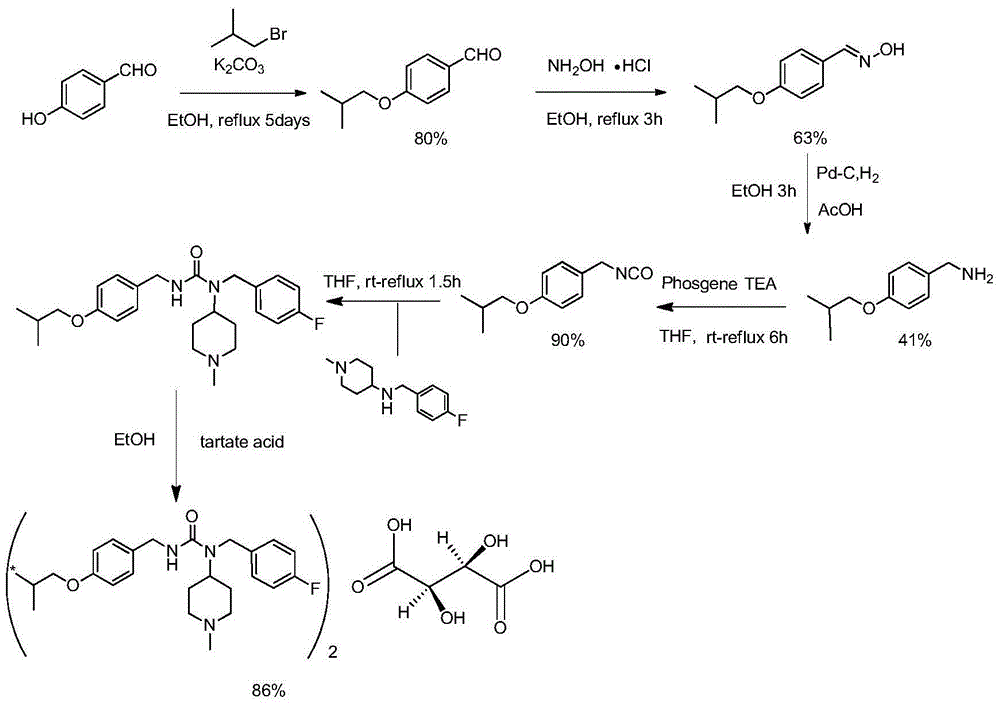
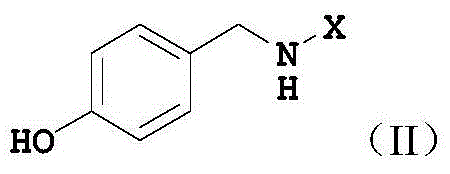
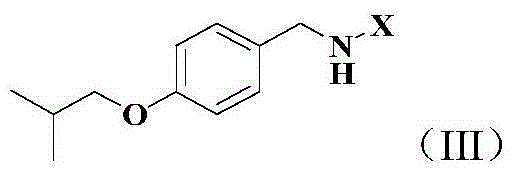
Synthetic method of tartrate of N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-isobutoxybenzyl)urea
A technology of isobutoxybenzyl and isobutoxybenzylamine, which is applied in the preparation of amino hydroxyl compounds, preparation of carbamic acid derivatives, chemical instruments and methods, etc., can solve the problem of long production cycle, complicated post-processing, and poor safety and other issues, to achieve the effect of less three wastes, easy to obtain raw materials, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, with ethoxycarbonyl as protecting group
[0050] 1) N-(4-hydroxybenzyl) ethyl carbamate
[0051] 4-Hydroxybenzylamine (20 g) and sodium bicarbonate (16 g) were added to dichloromethane (100 ml) and stirred at room temperature, and ethyl chloroformate (19.4 g) was added dropwise to in suspension. After the addition was complete, the mixture was stirred at 20°C to 25°C for 2 hours, starting material remaining by TLC. After the reaction was completed, suction filtration was performed to remove excess sodium salt. The filter cake was washed with dichloromethane (50ml x 2). The filtrate was washed with 5% dilute hydrochloric acid (36ml×2), and then washed with water (75ml×2). After separating the layers, the aqueous layer was discarded, and the organic layer was dried. The solvent was recovered under reduced pressure at 30°C to 40°C to obtain a crude red solid (30g), which was washed with toluene (45ml×2). The product was filtered and dried under vacuum at...
Embodiment 2
[0062] Embodiment 2, with methoxycarbonyl as protecting group
[0063] 1) Methyl N-(4-hydroxybenzyl)carbamate
[0064]Add 4-hydroxybenzylamine (15g) and sodium bicarbonate (15g) into dichloromethane (90ml) at room temperature (25°C, the same below) and stir, and dichloroformate formaldehyde at -25°C to 0°C for 1 hour The ester (13.8 g) was added dropwise to the suspension. After the addition was complete, the mixture was stirred at -25°C for 2 hours and the remaining starting material was checked by TLC. After the reaction was completed, suction filtration was performed to remove excess sodium salt. The filter cake was washed with dichloromethane (50ml x 2). The filtrate was washed with 5% dilute hydrochloric acid (36ml×2), and then washed with water (75ml×2). After separating the layers, the aqueous layer was discarded, and the organic layer was dried. The solvent was recovered under reduced pressure at 40°C to obtain a red liquid, which was dried under vacuum at 50°C for...
Embodiment 3
[0075] Example 3, using tert-butoxycarbonyl as a protecting group
[0076] 1) N-(4-hydroxybenzyl)-tert-butyl carbamate
[0077] 4-Hydroxybenzylamine (20g) and potassium bicarbonate (22g) were added to tetrahydrofuran (60ml) and water (15ml) at room temperature and stirred, di-tert-butyl dicarbonate (46.1 g) dropwise into the suspension. After the addition was complete, the mixture was stirred at 20°C to 25°C for 24 hours, starting material remaining by TLC. After the reaction was complete, the solid was filtered with suction, the filter cake was washed with tetrahydrofuran (30ml×2), the solid was washed with 5% dilute hydrochloric acid (36ml×2), the solid was filtered, washed with water (50ml×2), and filtered with suction. The solid was dried under vacuum at 60°C for 5 hours to obtain 14.7g of off-white powder, yield: 72.1%, mp: 105~107°C, ESI-MS m / z: 224[M+H] + ,246[M+Na] +
[0078] 2) tert-butyl N-(4-isobutoxybenzyl)carbamate
[0079] Dissolve N-(4-hydroxybenzyl)-tert-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com