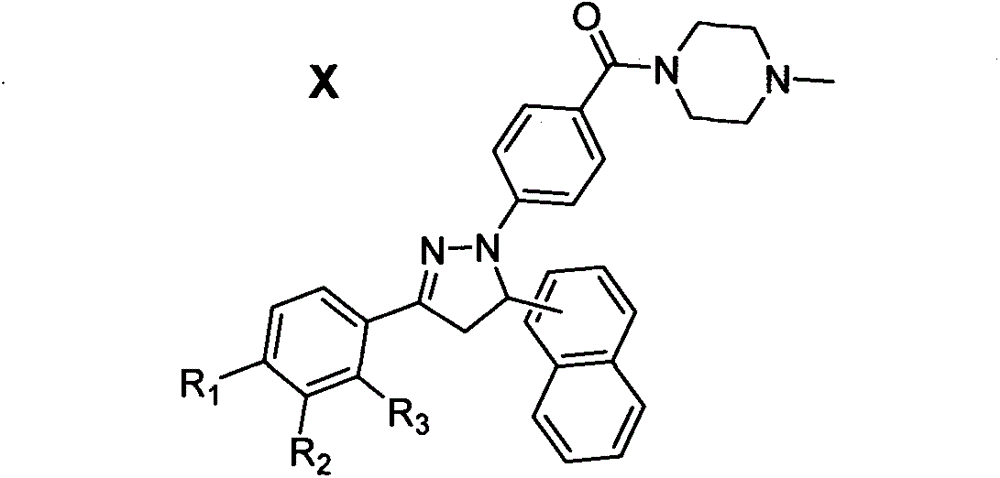
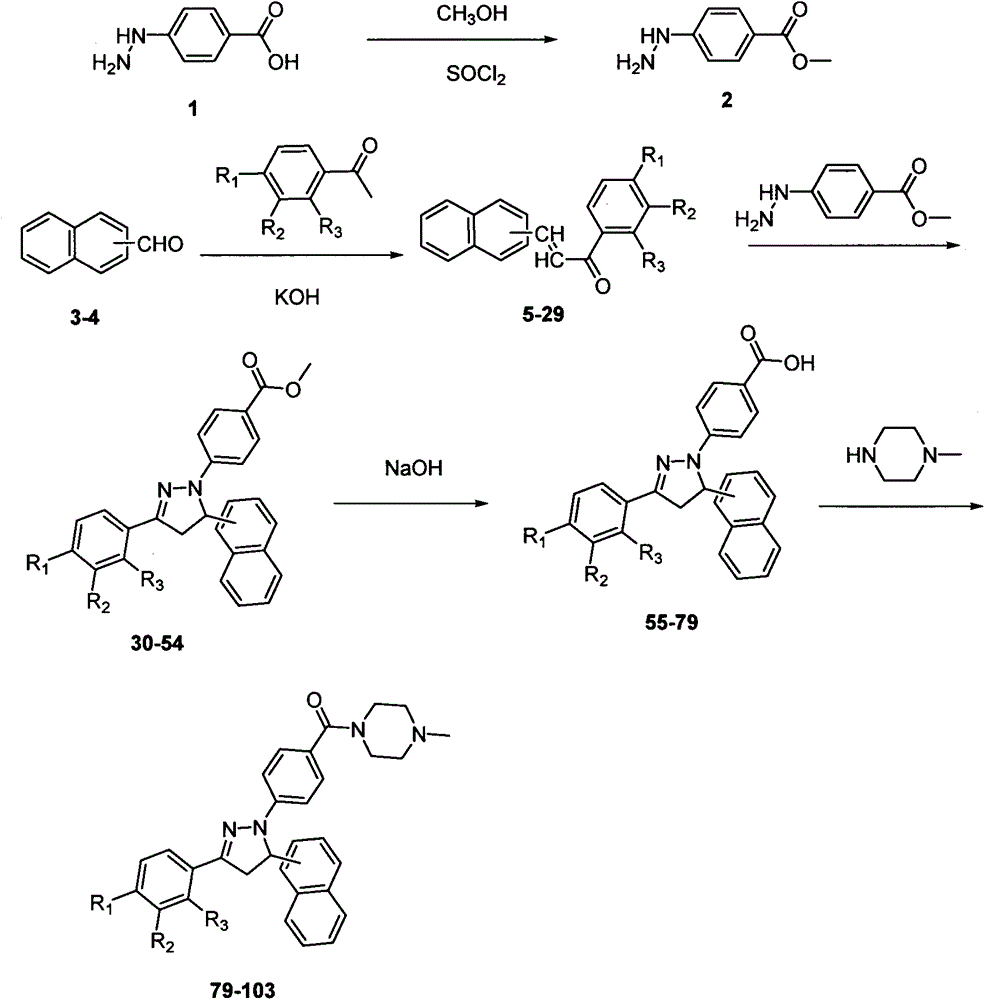
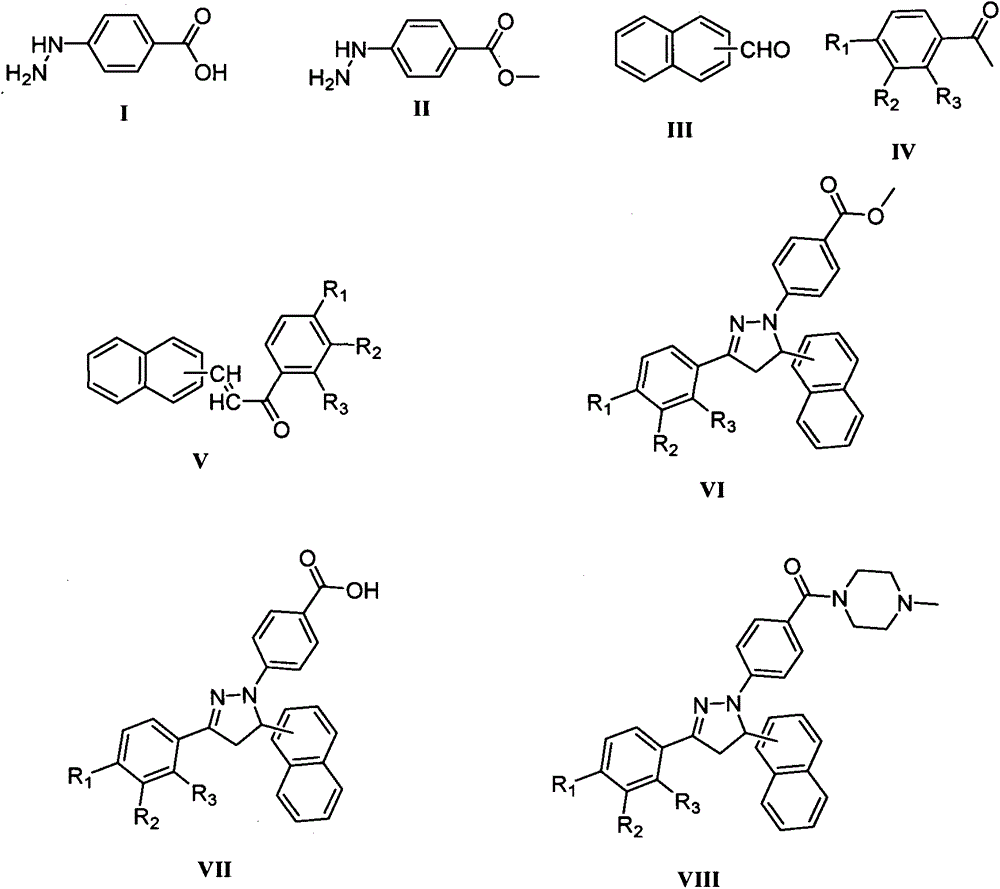
Preparation method of dihydropyrazol piperazine derivatives containing naphthalene ring skeleton and application in anti-cancer drugs
A technology of dihydropyrazole piperazine and derivatives, which is applied in the preparation of dihydropyrazole piperazine derivatives and the application field of anticancer drugs, can solve the problems of poor water solubility and limited clinical application, and achieve low toxicity, High reproducibility and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0027] A detailed embodiment of the present invention is as follows:
[0028] Step 1: Under the action of stirring at 0±5°C, dissolve the compound represented by formula I in anhydrous methanol in a round bottom flask, and add SOCl dropwise 2 , after 10±5min, transfer to 20±10°C and continue to stir for 6±3h, filter and dry, dissolve the obtained solid crude product in absolute ethanol and recrystallize to obtain the compound shown in formula II.
[0029] Step 2: Under stirring at 20±10°C, sequentially add the compound shown in formula III, the compound shown in formula IV and absolute ethanol to the round bottom flask, and add 5-50% NaOH aqueous solution drop by drop , after reacting for 4±2h, filter, and the obtained solid was washed with distilled water, cold ethanol, and distilled water successively, and dried. The obtained solid crude product was dissolved in absolute ethanol for recrystallization to obtain a compound with the structure shown in Formula V.
[0030] Step ...
Embodiment 1
[0034] Preparation of N-methylpiperazino(4-(5-(α-naphthyl)-3-phenyl-4,5-dihydropyrazole)phenyl)methanone (Compound 80)
[0035]
[0036] Under stirring at 0°C, sequentially add the intermediate 55, 4-dimethylaminopyridine, 1-hydroxybenzotriazole and anhydrous dichloromethane into the reaction vessel, and after 10±5 minutes, add 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, stirred and refluxed, TLC tracking reaction (developing agent V AcOEt :V 正己 烷 =2:1), after 8±3h, the reaction solution was sequentially washed with saturated KHSO 4 Aqueous solution, saturated Na 2 CO 3 , washed with saturated brine, and then rotary evaporated, and the obtained solid crude product was dissolved in absolute ethanol for recrystallization to obtain the target compound 80.
[0037] Crystals were obtained with a yield of 86.9%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.33(s, 1H, ArH), 8.02(d, J=7.9Hz, 1H, ArH), 7.87(d, J=8.3Hz, 1H, ArH), 7.78(d, J=6.8Hz , 2H, ArH), 7.70~7.58(m, ...
Embodiment 2
[0039] N-Methylpiperazino(4-(5-(α-naphthyl)-3-(4-methyl-phenyl)-4,5-dihydropyrazole)phenyl)methanone (Compound 81) preparation of
[0040]
[0041] The preparation method refers to Example 1. Crystals were obtained with a yield of 88.3%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.31(s, 1H, ArH), 8.01(d, J=7.9Hz, 1H, ArH), 7.86(d, J=8.2Hz, 1H, ArH), 7.68~7.59(m, 4H, ArH), 7.40(t, J=7.5Hz, 1H, ArH), 7.27~7.20(m, 5H, ArH), 6.94(d, J=8.3Hz, 2H, ArH), 6.22(s, 1H, CH) , 4.19 (dd, J 1 =12.6,J 2 =12.4Hz, 1H, CH), 3.54(s, 4H, CH 2 ), 3.45(s, 4H, CH 2 ), 3.36(s, 3H, CH 3 ), 3.08 (d, J=13.9Hz, 1H, CH 2 ), 2.18 (s, 3H, CH 3 ).ESI-MS: 489.6[M+H] + , C 32 h 32 N 4 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com