A method for immobilizing cells containing glucose isomerase
A glucose isomerase and cell technology, applied in the field of immobilization of glucose isomerase-containing cells, can solve the problems of weakened competitiveness, high production cost of HFCS, low conversion rate of HFCS, etc., and achieve satisfactory mechanical strength and immobilization method Simple and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Construction of Recombinant Escherichia coli E.coli BL21(DE3) / pET-28b-TEGI and Preparation and Performance Measurement of Wet Cells
[0032] The recombinant Escherichia coli E. coli BL21(DE3) / pET-28b-TEGI constructed in this laboratory is used as the production strain, and the method is as follows:
[0033] (1) Construction of recombinant bacteria: The heat-resistant GI gene TEGI of Thermoanaerobacter ethanolicus (the nucleotide sequence is shown in SEQ ID NO.1, which has been disclosed in Chinese patent 201410484596) and PGEM-T vector After ligation, it was introduced into E.coli JM109, TEGI / PGEM-T and plasmid pET28b(+)-Nit were double digested, ligated overnight with ligase, and the ligation product pET28b(+)-TEGI was introduced into the host E.coli BL21( In DE3), the recombinant Escherichia coli E. coli BL21(DE3) / pET28b(+)-TEGI was obtained.
[0034] The PGEM-T carrier connection conditions are as follows: 10 μL of the connection system is added to the PCR...
Embodiment 2
[0040] (1) Take the wet cells obtained by the method in Example 1, mix them according to the wet weight of the cells: Tris-HCl buffer solution (pH=7.5, 50mM)=1:10 (m / v), then take 10g of wet cells and add Prepare 100mL bacterial suspension in 100mL Tris-HCl buffer.
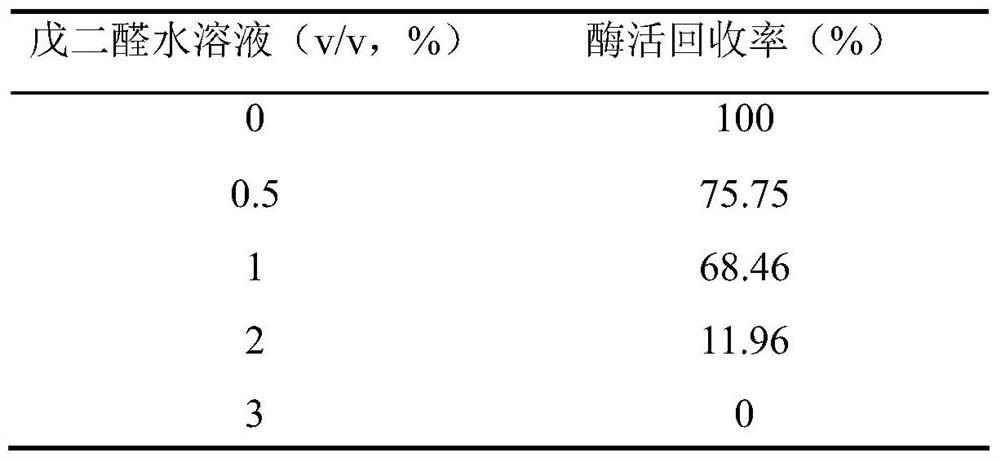
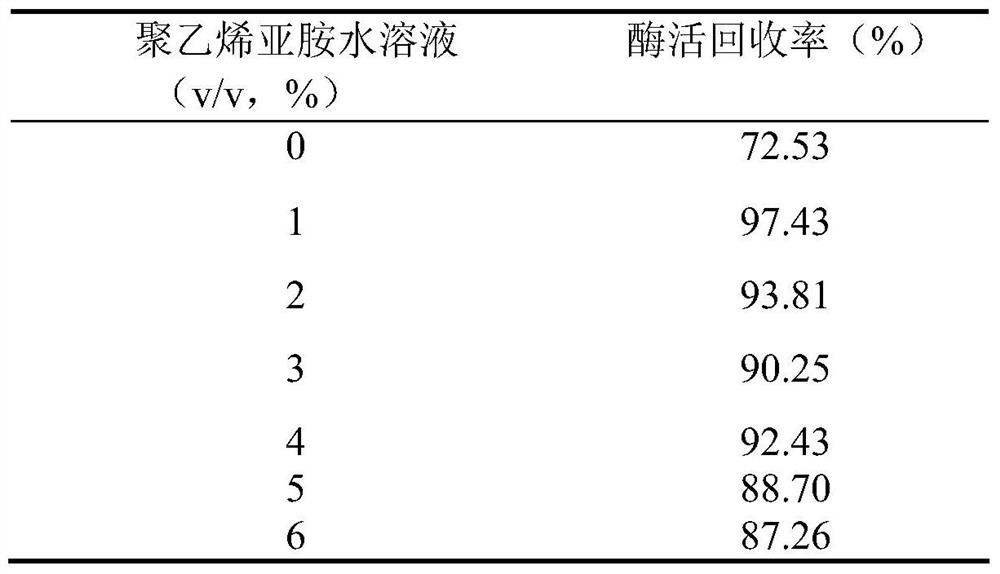
[0041] (2) Add 0.6g of diatomaceous earth to the 100mL bacterial suspension in step (1), mix evenly on a magnetic stirrer, and then add 1mL of polyethyleneimine aqueous solution with a concentration of 10% (v / v) It is 25% (v / v) glutaraldehyde aqueous solution. After cross-linking at 20°C for 2 hours, the reaction solution is suction-filtered. After the filter cake is washed with distilled water, it is extruded into long strips with an axial extruder and air-dried at room temperature. into small granules (0.5-2 mm in diameter), that is, immobilized cell granules containing GI. Under the same conditions, the order of adding polyethyleneimine and glutaraldehyde was reversed, that is, glutaraldehyde was added first, ...
Embodiment 3
[0045] (1) Take the wet thalline prepared by the method of Example 1, and mix it according to the wet weight of the thalline: Tris-HCl buffer solution (pH=7.5, 50mM)=1:10 (m / v) to obtain a bacterial suspension, that is, take 10g Wet cells were added to 100 mL Tris-HCl buffer to make 100 mL bacterial suspension.
[0046] (2) Add 0.6 g of diatomaceous earth to the 100 mL bacterial suspension in step (1), mix evenly on a magnetic stirrer, then mix with 3 mL of polyethyleneimine aqueous solution with a concentration of 10% (v / v), and then add 1.5 mL of glutaraldehyde aqueous solution with a concentration of 25% (v / v), after cross-linking at 20°C for 1.5 h, the reaction solution was filtered with suction, the filter cake was washed with distilled water, extruded into long strips with an axial extruder, and air-dried at room temperature Afterwards, small particles (0.5-2 mm in particle size) were made to obtain immobilized cells.
[0047] The residual enzyme activity test method is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com