Method of determining anti-thrombin activity of hirudin
A technology of antithrombin and determination method, which is applied in the field of medical inspection, can solve problems such as unsatisfactory repeatability and accuracy, standard curve error, and large environmental impact, and achieve low equipment requirements, good accuracy, and low detection concentration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
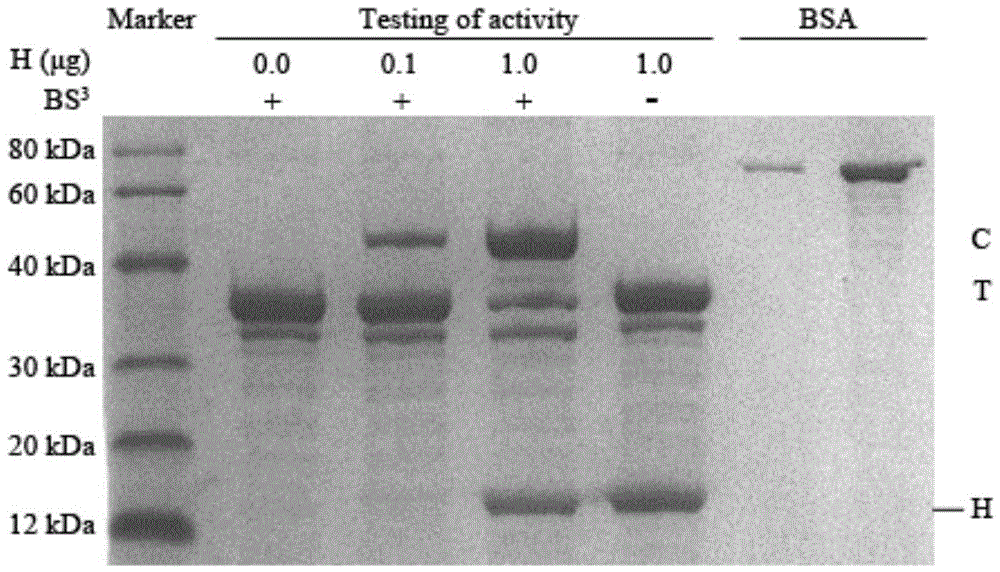
[0028] Add 11.5 μl, 10.5 μl, 1.5 μl, and 1.5 μl of binding buffer to the four tubes arranged from left to right, and then add 0 μl, 1 μl, 10 μl, and 10 μl of hirudin solution in sequence, and add them to the four tubes. Add 2 μl of thrombin solution respectively, after mixing, place the four tubes in a water bath at 37°C for 5 minutes, and add 1.5 μl of cross-linking agent BS to the first three tubes from left to right 3 Solution, add 1.5uL cross-linking buffer solution to the fourth tube, after cross-linking reaction for 30min, add 1uL stop buffer solution to the four tubes, react at room temperature for 15min to stop cross-linking, then add 4uL of 5 % SDS-PAGE protein loading buffer, after mixing, take 10uL sample from each tube for SDS-PAGE analysis and optical density scanning, and use 0.1ug and 1.0ug of bovine serum albumin (BSA) as a control, the gel Electrophoresis was stained with Coomassie brilliant blue to obtain figure 1 The electropherogram, wherein, the described...
Embodiment 2
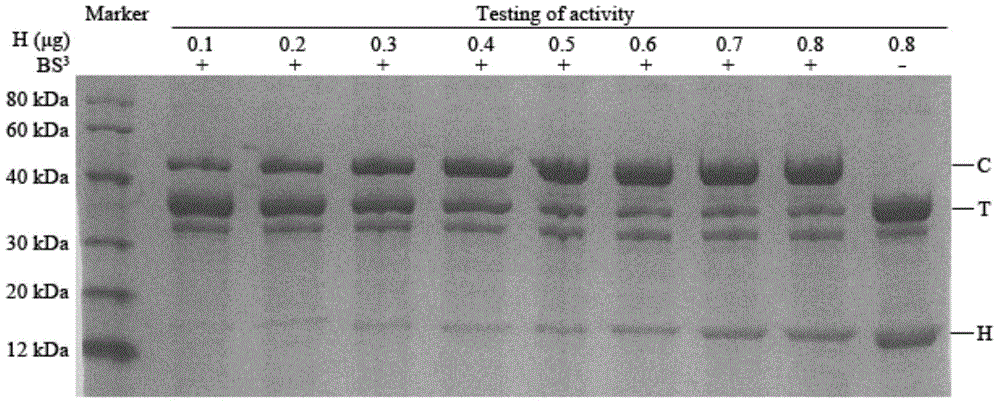
[0033] Step 1: Add 10.5 μl, 9.5 μl, 8.5 μl, 7.5 μl, 6.5 μl, 5.5 μl, 4.5 μl and 3.5 μl of binding buffer to the tubes marked 1-8 in sequence, then add 1 μl and 2 μl of hirudin solution in sequence , 3 μl, 4 μl, 5 μl, 6 μl, 7 μl and 8 μl, add 2 μl thrombin solution to 8 tubes respectively, after mixing, place them in a water bath at 37°C for 5 minutes, the binding buffer is a mass fraction of 0.9 % sodium chloride solution; the concentration of the hirudin solution is 0.2 mg / mL, and the thrombin solution is 4.8 mg / mL with a mass fraction of 0.9% sodium chloride solution as the solvent. 3NIH / uL solution;
[0034] Step 2: Add 1.5uL cross-linking agent BS to 8 tubes respectively 3 Solution, cross-linking reaction 30min at room temperature, the cross-linking agent BS 3 The solution consists of 12.5mmol BS 3 A solution obtained by dissolving 20mmol sodium phosphate in 1.5uL 0.9% sodium chloride solution;
[0035] Step 3: Add 1uL of stop buffer solution to the 8 tubes respectively...
Embodiment 3
[0041] On the basis of Example 2, after the samples in the 8 tubes were analyzed by SDS-PAGE, 0.1 ug and 1 ug of bovine serum albumin were used as controls for scanning densitometric detection.
[0042] The masses of the corresponding hirudin-thrombin compounds in the 8 tubes detected by optical density scanning were 0.45 μg, 0.91 μg, 1.36 μg, 1.82 μg, 2.27 μg, 2.4 μg, 2.4 μg and 2.4 μg, marked by 6 The quality of hirudin-thrombin compound in the tube of -8 can draw the quality of effective thrombin to be 2.0ug (being 5 / 6 of 2.4ug), therefore draw the mass fraction of effective thrombin in the thrombin solution to be 41.7 %(2.0 / 4.8×100%), since the hirudin in the tube labeled 1-5 is completely reacted, the activity of hirudin antithrombin in the tube labeled 1-5 is thus 5625ATU / mg . Antithrombin activity was calculated as follows:
[0043] The mass of hirudin-thrombin compound is: 0.45 μg;
[0044] The mass of effective thrombin is: 0.45×5 / 6=0.375ug;
[0045] The mass of h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com