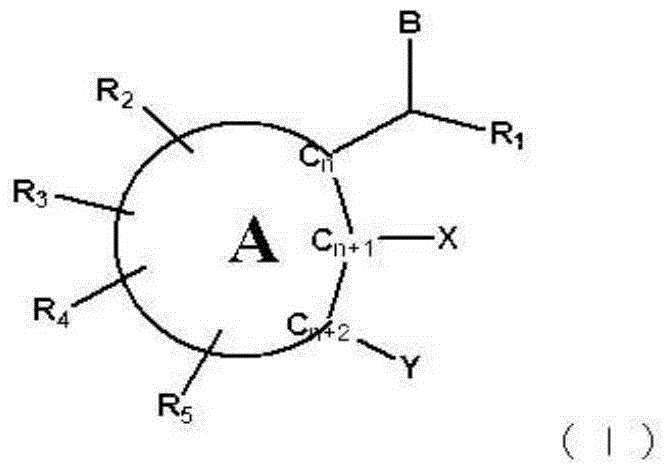
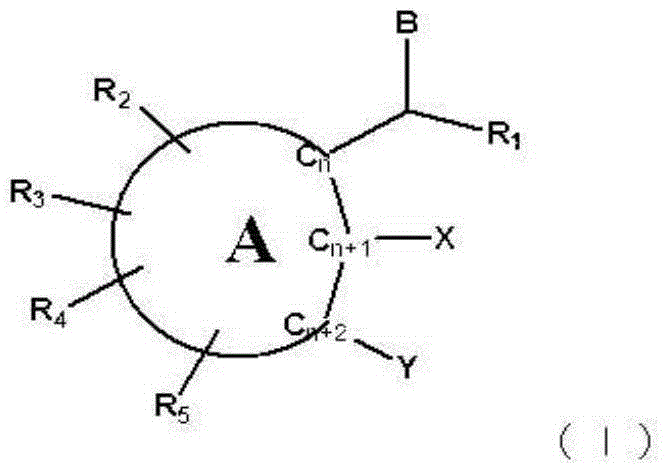
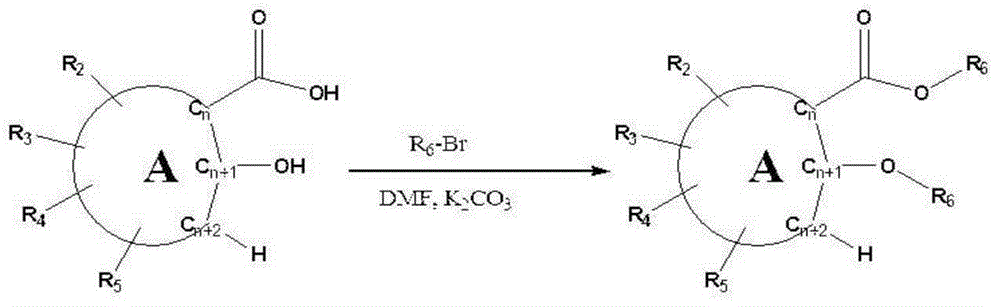
Skin whitening agent containing novel cyclic compound
A technology of heterocyclic compounds and compounds, applied in skin care preparations, medical preparations containing active ingredients, skin diseases, etc., can solve the problems of ascorbic acid oxidation, changes, difficulty in maintaining product consistency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] The preparation of embodiment 1.2-ethoxy ethyl benzoate
[0119] Into a round bottom flask with two openings was poured 10 mmol of 2-hydroxybenzoic acid at room temperature. Then, 50ml of dimethylformamide was added to the flask, and 24mmol of potassium carbonate (K 2 CO 3 ). Then 24 mmol ethyl bromide was slowly added dropwise thereto. After the dropwise addition was completed, the mixture was allowed to react at room temperature for 6 hours. 500ml of purified water was added to the reactant, and extracted with 100ml of n-hexane. After drying the hexane layer under reduced pressure, separation and purification were performed using silica gel column chromatography (ethyl acetate:hexane=1:50) to produce ethyl 2-ethoxybenzoate. The obtained product was identified by fast atom bombardment mass spectrometry (FAB-MS).
[0120] FAB quality: 195[M+H] +
Embodiment 2
[0121] The preparation of embodiment 2.2-propoxypropyl benzoate
[0122] Propyl 2-propoxybenzoate having the following physical properties was synthesized and obtained according to the same procedure as described in Example 1, except that bromopropane was used instead of bromoethane.
[0123] FAB quality: 223[M+H] +
Embodiment 3
[0124] The preparation of embodiment 3.2-butoxybutylbenzoate
[0125] Butyl 2-butoxybenzoate having the following physical properties was synthesized and obtained according to the same procedure as described in Example 1, except that bromobutane was used instead of bromoethane.
[0126] FAB quality: 251[M+H] +
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com