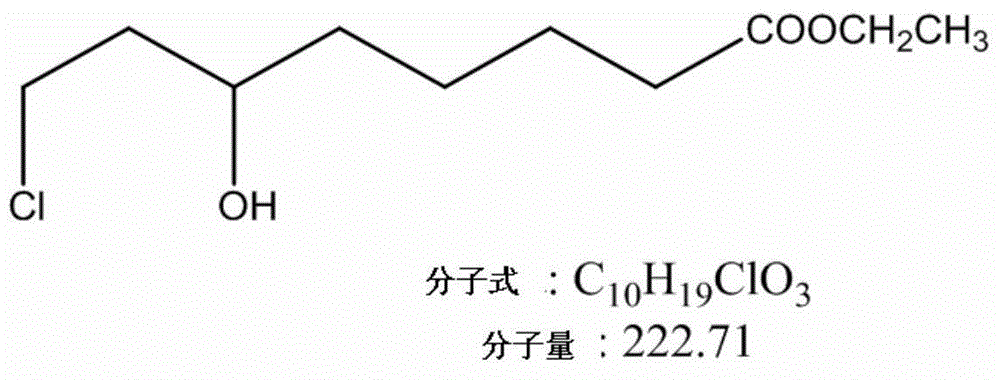
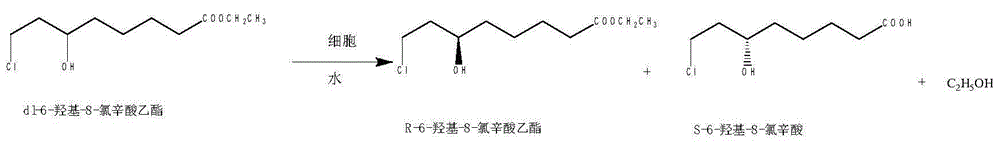
Method for preparing (S)-6-hydroxy-8-chlorine ethyl caprylate through reductase
A technology of chlorooctanoic acid ethyl ester and reductase, which is applied in the direction of fermentation, etc., can solve the problems of failure to increase yield, difficulty in obtaining high-purity R-lipoic acid, and ineffective utilization of lipoic acid, so as to meet the environmental protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] First, add 6-carbonyl-8-chlorooctanoic acid ethyl ester, ethanol, pH 7 tris(hydroxymethyl)aminomethane hydrochloride (i.e. Tris-HCl, i.e. Lewis acid, The same below) and glucose, the weight ratio of mixing ethyl 6-carbonyl-8-chlorooctanoate, glucose, tris(hydroxymethyl)aminomethane hydrochloric acid and ethanol is 1:2:5:2, and the stirring device is turned on and stirred to Glucose is fully dissolved, and then lactate dehydrogenase and NADP are added to the reaction vessel + (nicotinamide adenine dinucleoside phosphate, the same below) to react, mixing ethyl 6-carbonyl-8-chlorooctanoate, lactate dehydrogenase and NADP + The weight ratio of 10:0.8:0.006, and the control reaction temperature is 20 ℃, the control reaction time is 40h, in the reaction process, the pH value of the buffer of the reaction system is controlled by 0.1mol / L sodium hydroxide solution to pH 7, use organic solvent to extract repeatedly with toluene after the reaction finishes, combine organic phase...
Embodiment 2
[0026] First, add 6-carbonyl-8-chlorooctanoic acid ethyl ester, ethanol, pH 8 tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) and glucose in sequence to the reaction vessel equipped with stirring device, and vortex 6 - the weight ratio of carbonyl-8-chlorooctanoic acid ethyl ester, glucose, tris(hydroxymethyl)aminomethane hydrochloric acid and ethanol is 1:4:20:5, and the stirring device is turned on and stirred until the glucose is fully dissolved, and then in the reaction vessel Add lactate dehydrogenase and NADP + To perform the reaction, swirl ethyl 6-carbonyl-8-chlorooctanoate, lactate dehydrogenase, and NADP + The weight ratio is 10:0.9:0.004, and the control reaction temperature is 40 ℃, the control reaction time is 5h, in the reaction process, the pH value of the buffer of the reaction system is controlled at pH 8 with 2mol / l potassium hydroxide solution , after the reaction was completed, the organic solvent was used for multiple extractions with toluene, an...
Embodiment 3
[0028] First, add 6-carbonyl-8-chlorooctanoic acid ethyl ester, ethanol, pH 6 tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) and glucose in sequence to the reaction vessel equipped with stirring device, The weight ratio of 6-carbonyl-8-chlorooctanoic acid ethyl ester, glucose, tris(hydroxymethyl)aminomethane hydrochloric acid and ethanol is 1: 2: 25: 3, and the stirring device is opened and stirred until the glucose is fully dissolved, and then the reaction vessel is Lactate dehydrogenase and NADP are added to + To perform the reaction, swirl ethyl 6-carbonyl-8-chlorooctanoate, lactate dehydrogenase, and NADP +The weight ratio of 10:1.5:0.008, and the control reaction temperature is 30 ℃, the control reaction time is 24h, in the reaction process, the pH value of the buffer of the reaction system is controlled at pH 6 with ammonia solution, and after the reaction is completed Extracted with organic solvent ethyl acetate for several times, combined the organic phases ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com