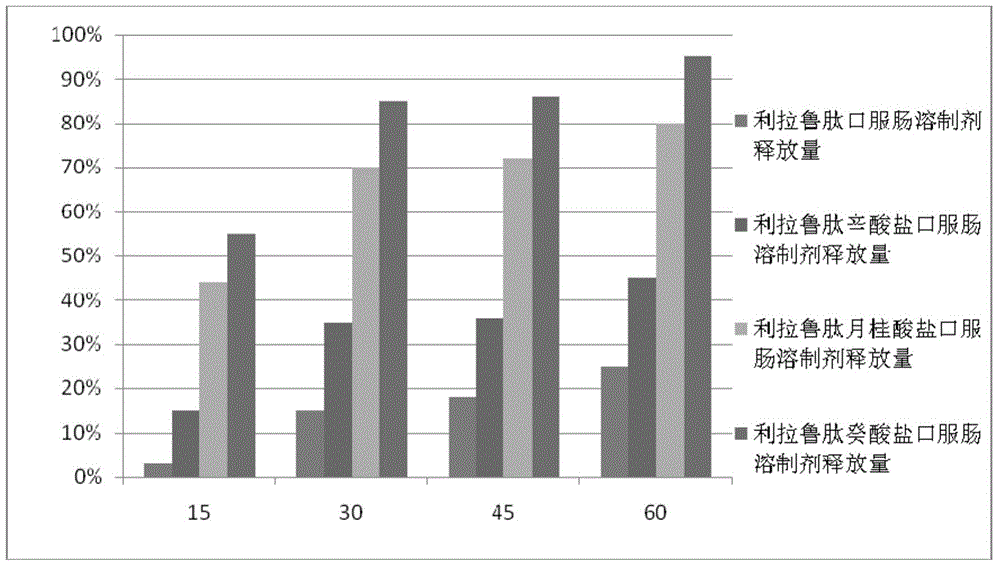
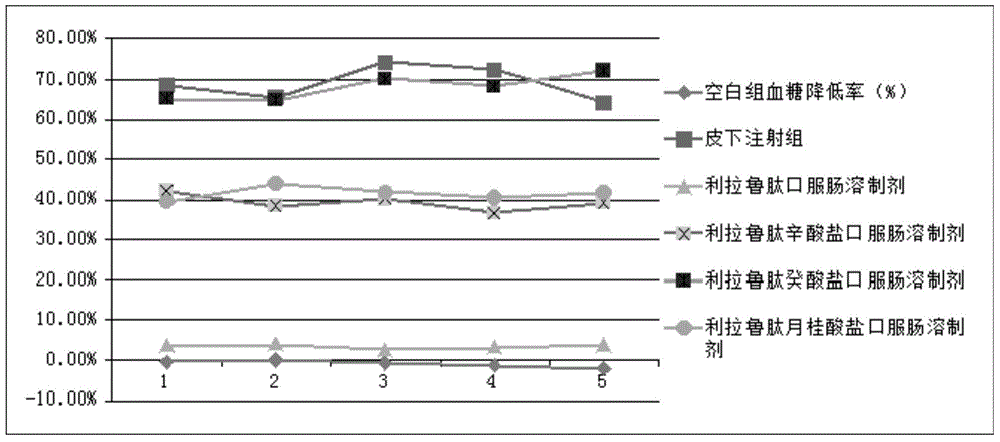
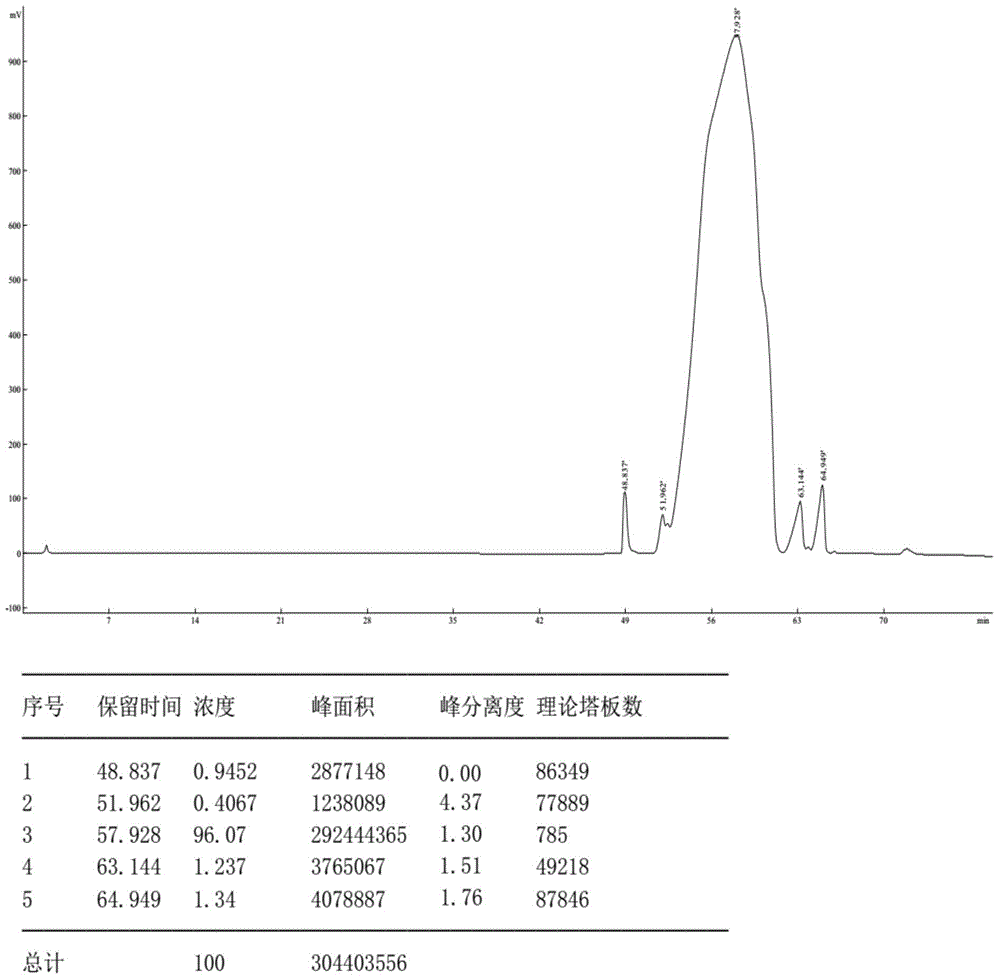
Intestinal absorption promoting liraglutide salt for preparing oral enteric-coated preparations
A technology of liraglutide salt and liraglutide, which is applied in the field of liraglutide salt to achieve the effects of improving blood drug concentration, insignificant hypoglycemic rate, and suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1, Preparation of Liraglutide Sodium Caprate, Liraglutide Sodium Laurate, and Liraglutide Sodium Caprylate
[0054] According to the patent method that our company has applied for (public application number: CN 103864918 A), the crude product of liraglutide was synthesized, and the crude product was purified by preparative liquid chromatography, concentrated by rotary evaporation to obtain a high-purity liraglutide concentrated solution, and then used The mobile phase containing sodium caprate is subjected to salt conversion treatment on the preparation liquid phase to obtain a sodium liraglutide caprate solution, which is concentrated by rotary evaporation and then freeze-dried to obtain sodium liraglutide caprate.
[0055] The preparation process of liraglutide sodium caprate is as follows:
[0056] An aqueous solution of sodium caprate with a concentration of 0.1% was prepared as mobile phase A, and acetonitrile was used as mobile phase B to be used for salt ...
Embodiment 2
[0086] Example 2 Oral enteric-coated preparation of liraglutide sodium caprate
[0087] main formula
[0088] Liraglutide Sodium Caprate 1g
[0089] Trehalose 5g
[0090] Preparation:
[0091] 1) Add the prescribed amount of microcrystalline cellulose and talcum powder at a weight ratio of 8:1, and prepare blank pellet cores with a centrifugal granulator.
[0092] Weigh the prescribed amount of microcrystalline cellulose and talcum powder, mix them with a three-dimensional mixer for 30 minutes, take half of them and put them in the centrifugal pellet machine, and put the other half in the feeder, and take an appropriate amount of purified water and put them in the liquid supply tank , turn on the centrifugal pellet making machine, turn on the turntable, air supply, and spray, wait until the material on the turntable is made into pellets, turn on the powder supply, until the material in the feeder is used up, turn off the spray, powder supply, discharge, and finally turn off...
Embodiment 3
[0099] Example 3 Preparation of oral enteric-coated preparations of liraglutide sodium caprylate
[0100] main formula
[0101] Liraglutide Octanoate Sodium 1g
[0102] Trehalose 5g
[0103] Preparation
[0104] 1) Add the prescribed amount of microcrystalline cellulose and talcum powder at a weight ratio of 8:1, and prepare blank pellet cores with a centrifugal granulator.
[0105] Weigh the prescribed amount of microcrystalline cellulose and talcum powder, mix them with a three-dimensional mixer for 30 minutes, take half of them and put them in the centrifugal pellet machine, and put the other half in the feeder, and take an appropriate amount of purified water and put them in the liquid supply tank , turn on the centrifugal pellet making machine, turn on the turntable, air supply, and spray, but for small pellets made of materials on the turntable, turn on the powder supply until the material in the feeder is used up, turn off the spray, powder supply, discharge, and fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com