Method for synthesizing chiral cyclic amine through catalyzing asymmetric hydrogenation of quinolin-3-amine by chiral phosphoric acid
A technology of phosphoric acid-catalyzed quinoline and chiral exocyclic amines, applied in the direction of organic chemistry, etc., to achieve the effects of complete conversion of raw materials, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
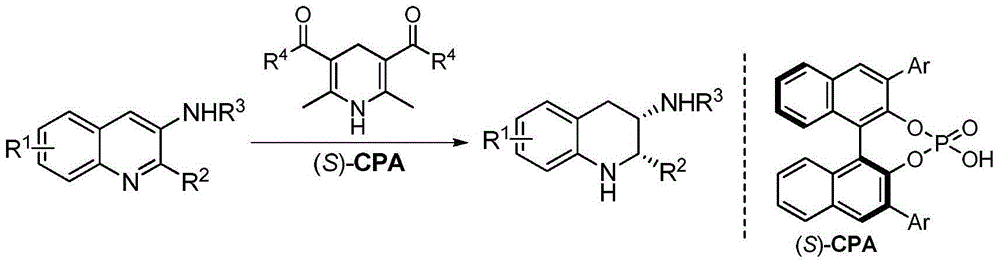
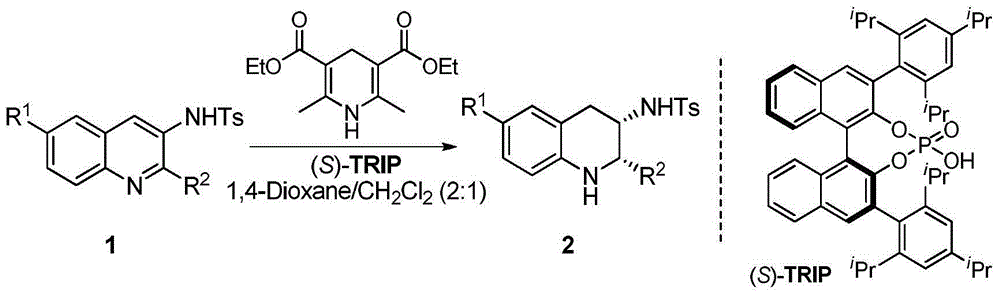
[0026] Example: Chiral phosphoric acid catalyzed asymmetric transfer hydrogenation of quinoline-3-amine to synthesize various chiral exocyclic amine compounds
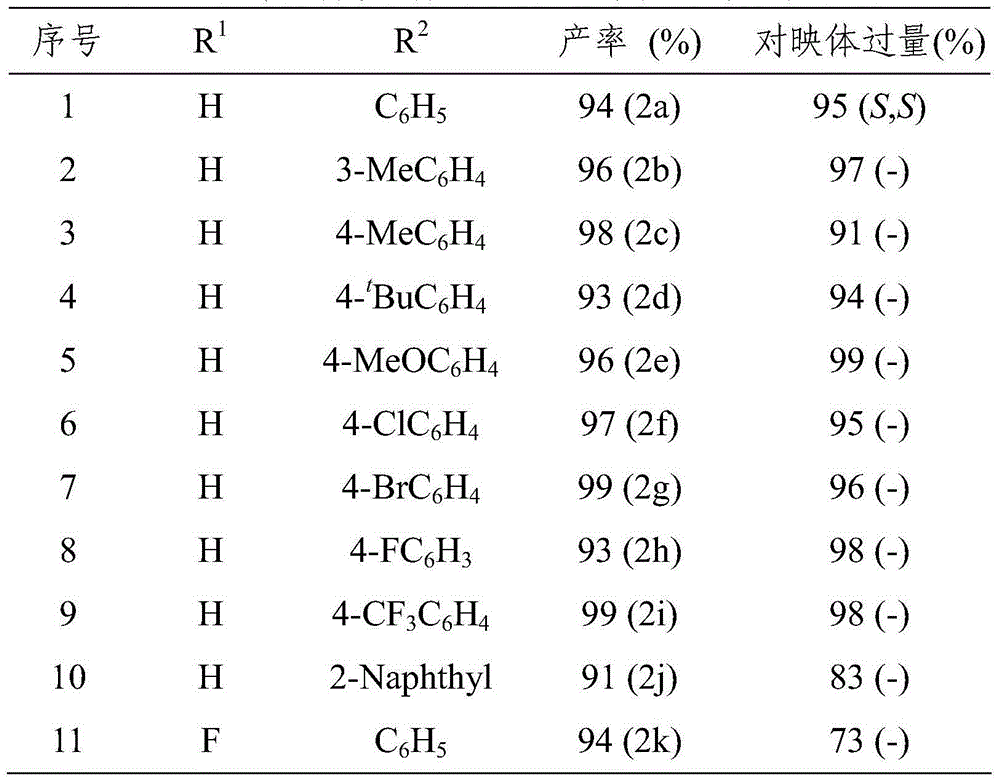
[0027] Under nitrogen protection, add quinoline-3-amine (1), Hantzsch ester (2.4eq.) and chiral phosphoric acid ((S)-TRIP, 5mol%) into the Schlenk tube with a volume ratio of 2:1 3 milliliters of mixed solvents of 1,4-dioxane and dichloromethane, stirred and reacted at 25 ℃ for 24 hours afterwards, column chromatography (eluent: sherwood oil and ethyl acetate) after removing solvent with rotary evaporator The volume ratio is 10:1-5:1) to obtain pure product, and the reaction formula is as follows.
[0028]
[0029] 4-Methyl-N-((2S,3S)-2-phenyl-1,2,3,4-tetrahydroquinolin-3-yl)benzonesulfonamide(2a):94%yield,95%ee,white solid,mp175- 177℃,[α] 20 D =+73.2(c0.88,CH 2 Cl 2 ), R f =0.40(petroleum ether / EtOAc5:1). 1 H NMR (400MHz, CDCl 3 )δ=7.35(d,J=8.2,2H),7.25-7.17(m,5H),7.04(t,J=8.8,3H),6.93(d,J=7.4,1H),6.72(t,J ...
Embodiment 2
[0043] Embodiment 2: the synthesis of a kind of substance P antagonist 4
[0044] Under the protection of nitrogen, under the condition of vigorous stirring, add naphthalene (2.00 mmol) to the 4 mL tetrahydrofuran solution added with Na (2.00 mmol), after 1 hour, transfer it to a constant pressure dropping funnel, Under the condition of ℃, drop it into 4mL tetrahydrofuran solution dissolved with 2a (0.10 mmol), after the reaction is complete (about 1 hour), stop the reaction, add 10mL water to quench the reaction, separate the layers, and extract the aqueous phase with dichloromethane, The organic phases were combined, dried with anhydrous sodium sulfate, filtered, and spin-dried, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and then the solvent was removed by rotary evaporation, and the pure product 3 was obtained by column chromatography.
[0045] Under nitrogen protection, compound 3 (0.10 mmol) and NaBH (OAc) 3 (0.30 mmol) was dissolved i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com