Triaryl sulfonium salt containing benzoxazole skeleton and preparation method thereof
A technology of benzoxazolylsulfenyl and benzoxazole, which is applied in the field of triarylsulfonium salts, can solve the problems of low solubility, achieve high conjugation degree, high conjugation degree, and reduce the release amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
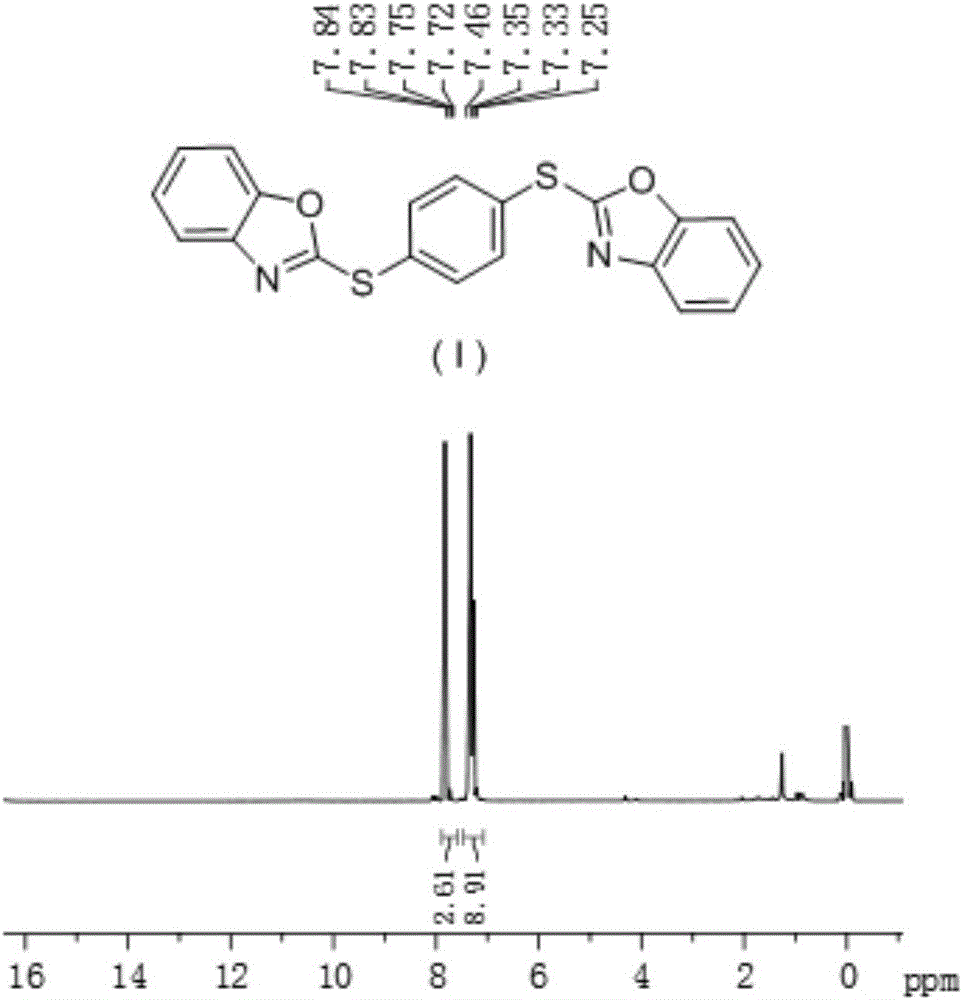
[0025] In a 100mL long-necked round bottom flask, add 2-mercaptobenzoxazole (2mmol), 1,4-diiodobenzene (1mmol) successively, dissolve with DMF (4mL), and then add cuprous iodide (0.1mmol) , and finally potassium hydroxide (4 mmol) was added. Put the flask into a microwave reactor, set the power to 30W, and react for 20min. The reaction solution was transferred to a separatory funnel, extracted with ethyl acetate and washed with brine, then the organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, followed by column chromatography to obtain the product 1,4-bis(benzene and oxazol-2-thio)benzene, compound (I).
[0026] Compound (I): white crystals, m.p.125-128°C, yield 75.8%. 1 H NMR (CDCl 3 , 300MHz) δ:7.25-7.46(m,ArH,9H),7.72-7.84(m,ArH,3H).
Embodiment 2
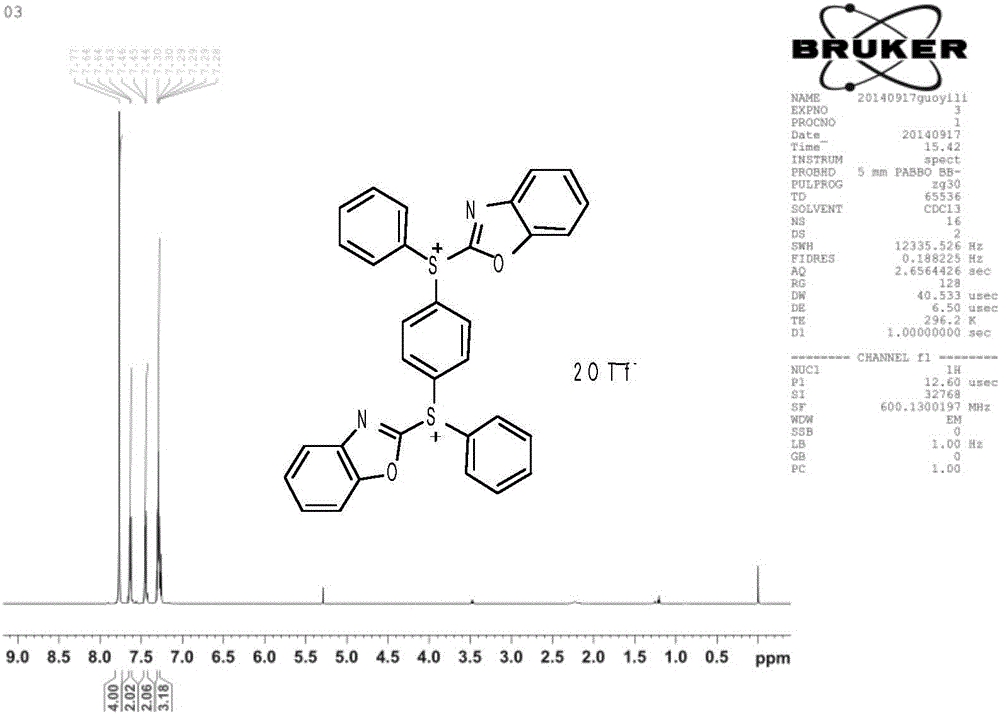
[0028] In a 50mL single-neck round bottom flask equipped with a stirring bar, the compound 1,4-bis(benzoxazol-2-thio)benzene (1mmol), diphenyliodotrifluoromethanesulfonate (2.4 mmol), dissolved in 1,1,2,2-tetrachloroethane (2 mL), and finally added cuprous iodide (0.1 mmol), stirred evenly, and reacted in an oil bath at 110° C. for 2 h. The reaction solution was transferred to a separatory funnel, extracted with ethyl acetate and washed with brine, then the organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, followed by column chromatography, and the mixture was sequentially washed with dichloromethane, ethyl acetate The ester was used as the eluent for column chromatography to obtain compound (II-a) as white crystals.
[0029]
[0030] Compound (II-a): white crystals, m.p 202-205°C, yield 66.7%. 1 HNMR (CDCl 3 , 300MHz) δ:7.28-7.30(m,ArH,3H),7.44-7.46(m,ArH,2H),7.63-7.64(m,ArH,2H),7.77(m,ArH,4H).
Embodiment 3
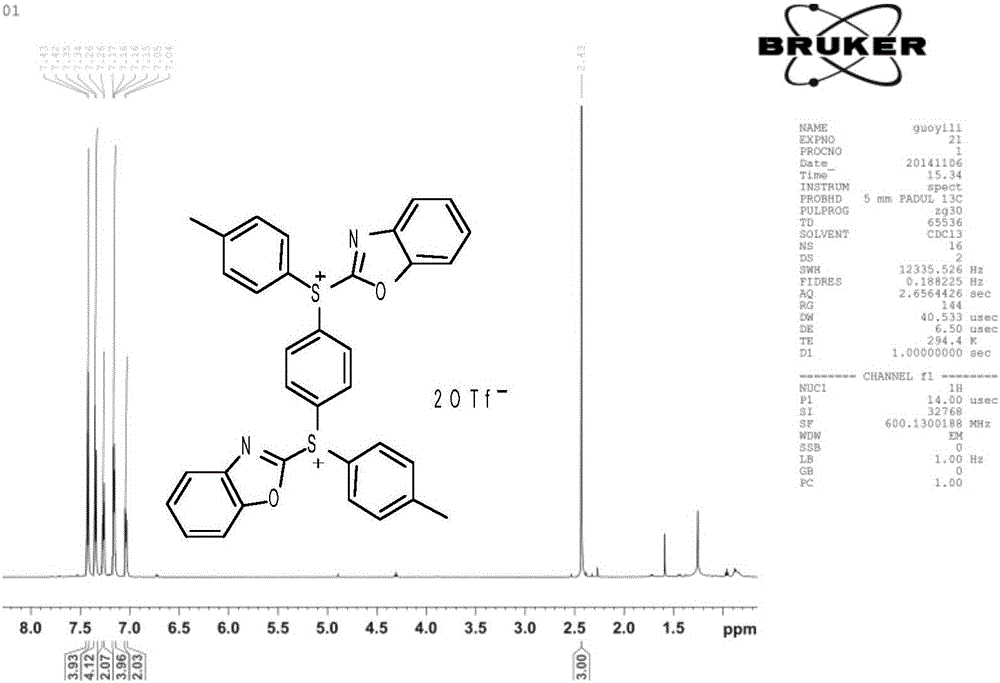
[0032] In a 50mL single-necked round bottom flask equipped with a stirring bar, the compound 1,4-bis(benzoxazol-2-thio)benzene (1mmol), bis-(4-methylphenyl)iodotrifluoromethane were sequentially added The sulfonate (2.4 mmol) was dissolved in 1,1,2,2-tetrachloroethane (2 mL) and finally copper iodide (0.1 mmol) was added. After stirring evenly, react in an oil bath at 110°C for 2h. The reaction solution was transferred to a separatory funnel, extracted with ethyl acetate and washed with brine, then the organic phase was dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, followed by column chromatography, and the mixture was sequentially washed with dichloromethane, ethyl acetate The ester was used as the eluent for column chromatography to obtain compound (II-b) as white crystals.
[0033]
[0034] Compound (II-b): white crystals, m.p 167-169°C, yield 76.1%. 1 H NMR (CDCl 3 ,300MHz)δ:2.43(s,CH 3 ,3H),7.04-7.05(m,ArH,2H),7.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com