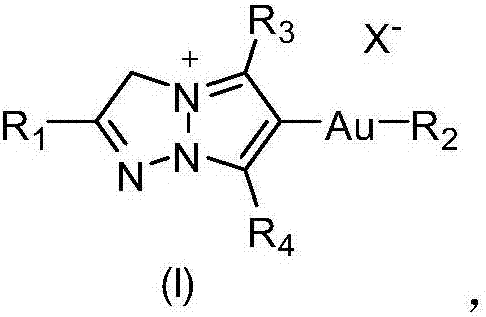
A kind of gold triazole compound and its preparation method and application
A compound, the technology of triazole gold, applied in the field of triazole gold compounds and the preparation thereof, can solve the problems of large-scale parallel synthesis of triazole compounds, producing false positives or false negatives, unable to become building units, etc., and achieves strong water solubility. , the effect of easy availability of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Ph 3 PAuCl was dissolved in dry dichloromethane, an equimolar amount of silver salt was added, and stirred at 10°C for 3 minutes. The mixed solution was filtered through a short column of diatomaceous earth to remove insoluble matter to obtain a clear solution, which was then added dropwise to 2-(1,3-diphenylprop-2-yn-1-yl)-4- In the dichloromethane solution of phenyl-2H-1,2,3-triazole, it stirred at 25 degreeC for 1 hour. Concentrate the solution with a rotary evaporator, then add n-hexane, a white precipitate appears, and filter to obtain a white solid, which is further recrystallized with a 2:1 dichloromethane:n-hexane solution to obtain white crystal 1 with a yield of 90.1%. 1 H NMR (600MHz, CDCl 3 ): δ8.33-8.37(m, 4H), 8.09(d, J=7.8Hz, 2H), 7.47-7.57(m, 24H), 6.21(s, 2H); 13 CNMR (150MHz, CDCl 3 ): δ167.4, 150.0, 145.2, 134.15, 134.06, 133.8, 131.8, 131.1, 130.6, 129.7, 129.44, 129.42, 129.38, 129.31, 129.25, 128.8, 128.6, 128.1, 126.7, 5; 31 P NMR (242.7MHz, ...
Embodiment 2
[0037] Ph 3 PAuCl was dissolved in dry dichloromethane, 2 times the molar amount of silver salt was added, and stirred at 20°C for 20 minutes. The mixed solution was filtered through a short column of diatomaceous earth to remove insoluble matter to obtain a clear solution, which was then added dropwise to 2-(1,3-diphenylprop-2-yn-1-yl)-4- In the dichloromethane solution of phenyl-2H-1,2,3-triazole, it stirred at 30 degreeC for 2 hours. Concentrate the solution with a rotary evaporator, then add n-hexane, a white precipitate appears, filter to obtain a white solid, and further recrystallize with a 2:1 dichloromethane:n-hexane solution to obtain white crystal 1, and obtain a white powder with a yield of 88.1 %. 1 HNMR (600MHz, CDCl 3 ): δ8.35-8.38(m, 4H), 8.07(d, J=7.8Hz, 2H), 7.45-7.58(m, 24H), 6.11(s, 2H); 13 C NMR (150MHz, CDCl3 ): δ167.2, 150.1, 145.1, 134.35, 134.16, 133.7, 131.8, 131.0, 130.5, 129.6, 129.43, 129.4, 129.39, 129.33, 129.26, 128.8, 128.7, 128.0, 111.6, 1...
Embodiment 3
[0041] Ph 3 Dissolve PAuCl in dry dichloromethane, add 3 times molar amount of silver salt, and stir at 40°C for 30 minutes. The mixed solution was filtered through a short column of diatomaceous earth to remove insoluble matter to obtain a clear solution, which was then added dropwise to 2-(1,3-diphenylprop-2-yn-1-yl)-4- In the dichloromethane solution of phenyl-2H-1,2,3-triazole, it stirred at 20 degreeC for 1.5 hours. Concentrate the solution with a rotary evaporator, then add n-hexane, a white precipitate appears, filter to obtain a white solid, and further recrystallize with a 2:1 dichloromethane:n-hexane solution to obtain a white crystal 1, and obtain a white powder with a yield of 88.1 %. 1 H NMR (400MHz, CDCl 3 ):δ8.34(m,4H),7.96(d,J=8.2Hz,2H),7.56-7.48(m,21H),7.30(d,J=8.0Hz,2H),6.14(s,2H) ,2.38(s,3H); 13 C NMR (101MHz, CDCl 3 ):δ167.33,144.93,134.12,133.98,131.81,131.78,131.04,130.55,130.16,129.72,129.37,129.26,129.18,128.64,128.56,128.55,128.48,127.97,123.90,5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com