A kind of synthetic method of 4,4-dimethyl-1,3-dioxane
A synthetic method, the technology of dioxane, applied in 4 fields, can solve the problems of unsatisfactory reaction results, incomplete reaction, low product yield, etc., and achieve the effect of easy control of reaction, complete reaction and high reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
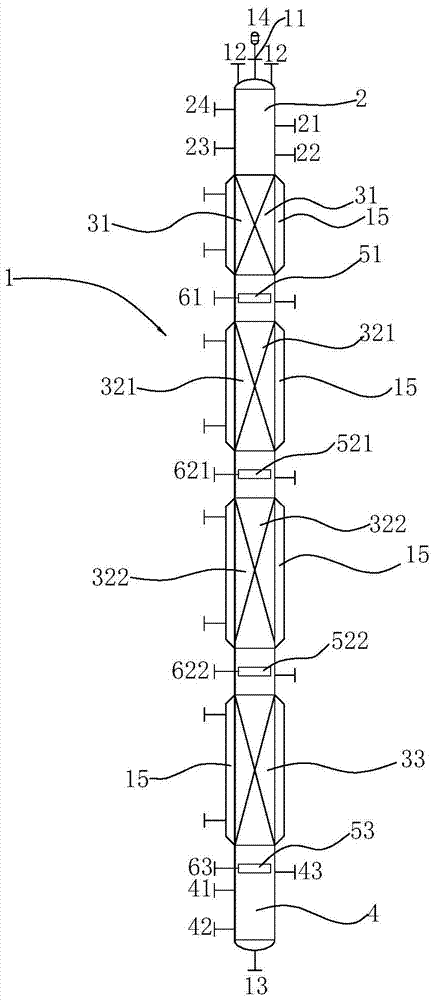
[0054] This 4,4-dimethyl-1, the synthetic method of 3-dioxane comprises the following steps:
[0055] Phosphoric acid aqueous solution enters the reactor from the upper inlet between the upper packing area and the first intermediate packing area;
[0056] The aqueous formaldehyde solution enters the reactor from the first intermediate inlet located at the first intermediate packing area and the second intermediate packing area;
[0057] The tert-butanol aqueous solution enters the reactor from the second intermediate inlet between the second intermediate packing area and the lower packing area;
[0058] Isobutene enters the reactor from the lower inlet located below the lower packing area;
[0059] Phosphoric acid solution and formaldehyde flow from top to bottom in the reactor; part of tert-butanol flows from top to bottom with the water phase in the reactor, and part flows from bottom to top with the oil phase; isobutene flows from bottom to top in the reactor, Countercurr...
Embodiment 1
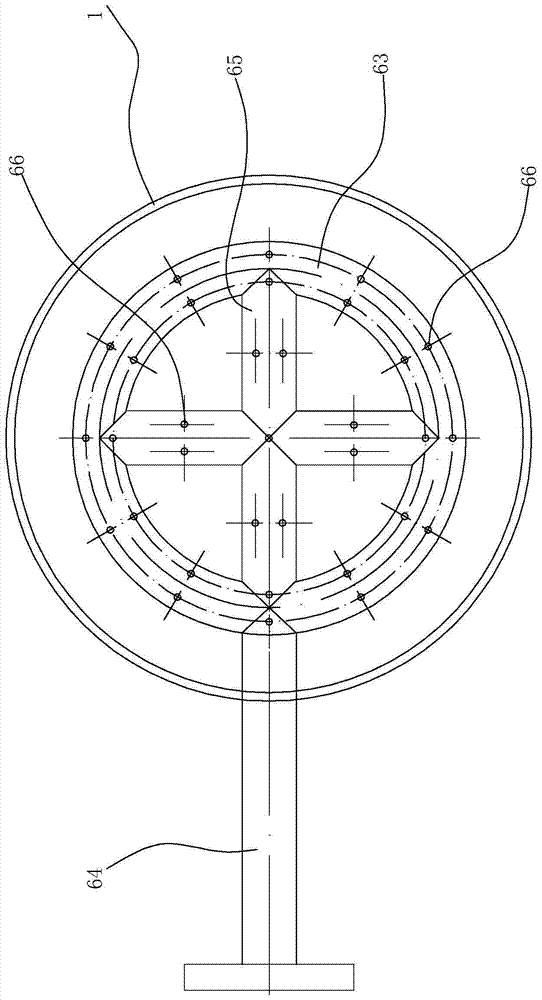
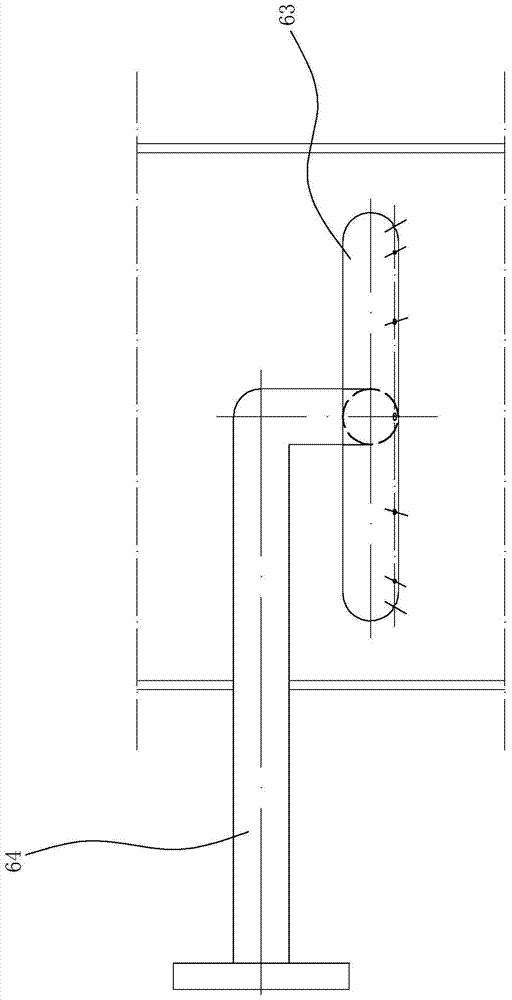
[0064] In this embodiment, the packing height of the lower packing area is 8m, the packing height of the second middle packing area is 1m, the packing height of the first middle packing area is 0.5m, and the packing height of the upper packing area is 0.5m; the packing is wound glass Glass springs are scattered in each packing area; the inner diameter of the reactor is
[0065] Isobutene: purity 99.9%; feed from the lower material distributor, flow rate 184g / h, feed temperature 70°C;
[0066] Tert-butanol aqueous solution: the content of tert-butanol is 85.1%, fed from the first intermediate material distributor, the flow rate is 220g / h, and the feed temperature is 85°C;
[0067] Formaldehyde aqueous solution: formaldehyde content 36.5%, methanol content 1.0%; flow rate 165g / h, feed temperature 86°C;
[0068] Phosphoric acid aqueous solution: phosphoric acid content 7.5%; flow rate 381g / h, feed temperature 86°C;
[0069] Formaldehyde aqueous solution and phosphoric ac...
Embodiment 2
[0075] Isobutene: purity 99.9%, fed from the lower feed distributor, flow rate 188g / h, feed temperature 71°C;
[0076] Tert-butanol aqueous solution: the content of tert-butanol is 85.1%, fed from the second intermediate feed distributor, the flow rate is 220g / h, and the feed temperature is 88°C;
[0077] Formaldehyde aqueous solution: formaldehyde content 36.5%, methanol content 1.0%, feed from the upper feed distributor, flow rate 140g / h, feed temperature 85°C;
[0078] Phosphoric acid aqueous solution: the phosphoric acid content is 7.2%, fed from the upper feed distributor, the flow rate is 369g / h, and the feed temperature is 85°C.
[0079] The first intermediate feed port is closed.
[0080] Control the reaction temperature in the reactor to be 85±5°C, and the reaction pressure to be 2.0±0.1MPa.
[0081] The outlet flow rate of the oil phase is 392g / h, and the outlet flow rate of the water phase is 500g / h.
[0082] The test results of oil phase and water phase are show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com