4'-desoxy-4'-alkylated or acylated amino avermectin B2a/2b derivative, and preparation method and application thereof
A technology of acylated amine group and avermectin, which can be used in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., and can solve problems such as unutilized B2a/2b
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
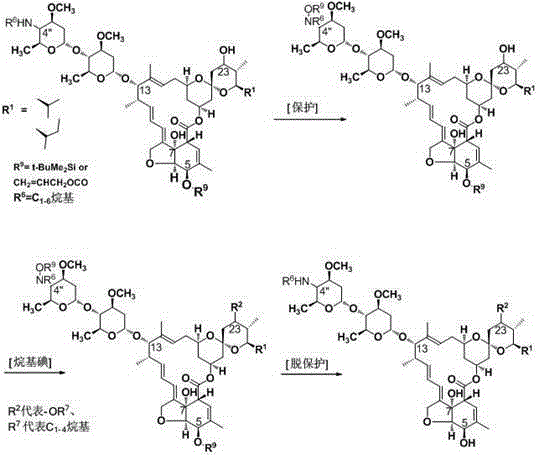
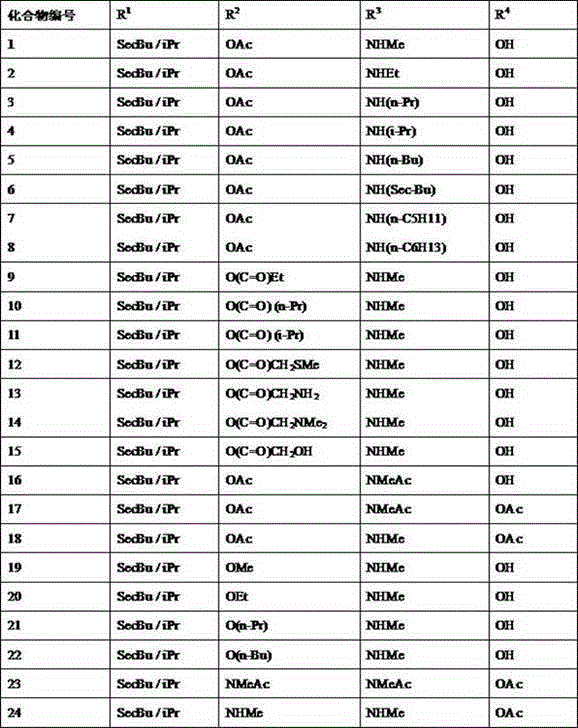
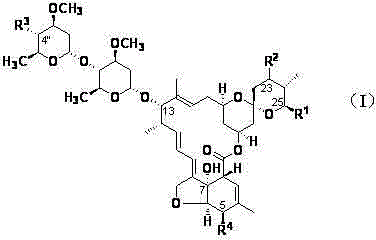
[0078] Example 14 "-deoxy-4"-methylamino-23-acetoxy abamectin B 2a / 2b preparation of
[0079] 10.0g 4”-carbonyl-5-protected abamectin B 2a / 2b Dissolve in 120g sec-butyl acetate, cool down to 10°C, add 3.0g triethylamine, add DMAP as catalyst, add dropwise acetic anhydride (1.5g acetic anhydride dissolved in 10g sec-butyl acetate), after 2h, heat up to 60-70°C, add 1g of zinc trifluoroacetate and 5g of heptamethyldisilazane, after 6h, lower the temperature of the reaction solution to -10°C, add methanol, and add 0.8g of NaBH in batches 4 , reacted for 1h, raised the temperature to 0-5°C, added 0.005g tetrakistriphenylphosphine palladium, added 1.0g sodium borohydride in batches, and reacted for 1h, removed the 5-position protecting group, and terminated the reaction with 20% acetic acid solution. Adjust PH=2-3, then adjust PH=7-8 with 10% sodium hydroxide solution, and separate layers. The aqueous phase was extracted twice with 20g of sec-butyl acetate, the organic phases we...
Embodiment 24
[0080] Example 24"-deoxy-4"-(N-acetylmethylamino)-23-acetoxy abamectin B 2a / 2b Preparation of (preferred compound)
[0081] 11.5g 4"-methylamino-5-protected abamectin B 2a / 2b Dissolve in 90g of dichloromethane, cool down to 0°C, add 4.0g of triethylamine, dropwise add 3.4g of acetic anhydride, react for 2h, cool down the reaction solution to 0-5°C, add 10g of methanol, 0.005g of tetrakistriphenylphosphine Palladium, add 1.0g sodium borohydride in batches, after 1 hour, stop the reaction with 20% acetic acid solution, adjust PH=2-3, then adjust PH=7-8 with 10% sodium hydroxide solution, and separate layers. The aqueous phase was extracted twice with 20g of dichloromethane, the organic phases were combined, heated and distilled under reduced pressure, and the vacuum degree was 0.09MPa for 1h to obtain 8.6g of white solid, namely: 4”-deoxy-4”-(N-acetylmethylamino) -23-Acetoxyabamectin B 2a / 2b , the content is 95.0%, and the yield is 83.4%.
[0082] l HNMR (CDCl 3 ,500Hz)σ8....
Embodiment 34
[0083] Example 34 "-deoxy-4"-methylamino-23-O(C=O)CH 2 NMe 2 Abamectin B 2a / 2b preparation of
[0084] 11.0g 4"-carbonyl-5-protected abamectin B 2a / 2b Dissolve in 100g isopropyl acetate, keep warm at 30°C, add 3.0g anhydrous sodium carbonate, add DMAP, drop Cl(C=O)CH 2 NMe 2 1.3g, after 2h, add heptamethyldisilazane and zinc trifluoroacetate, raise the temperature to 60°C, react for 6h, lower the temperature, lower the temperature of the reaction solution to -10°C, add methanol, add 0.8gNaBH4 in batches, and react 1h, heat up to 0-5°C, add 0.005g tetrakistriphenylphosphine palladium, add 1.0g sodium borohydride in batches, react for 1h, remove the 5-position protecting group, and terminate the reaction with 20% acetic acid solution after the reaction , adjust PH=2-3, then adjust PH=7-8 with 10% sodium hydroxide solution, and separate layers. The aqueous phase was extracted twice with 20 g of isopropyl acetate, the organic phases were combined, heated and distilled under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com