Synthetic method for lithium ion cathode material LiNiO2/C
A technology of positive electrode materials and synthesis methods, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve problems such as deterioration of electrochemical performance, poor thermal stability, and decrease in specific capacity, so as to prevent excessive grain production, Improve electrical conductivity and prevent agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
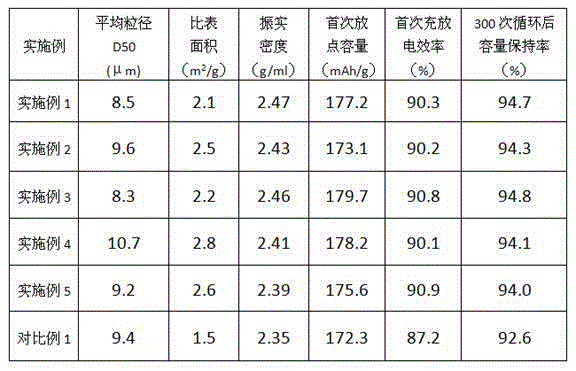
[0018] According to n(Li):n(Ni)=1.03, weigh 910 lithium acetate and 1000g nickel monoxide, add 100g organic carbon source glucose according to the weight of nickel monoxide 10%, add nickel monoxide and glucose, add 3L anhydrous salt water Mix evenly, ball mill for 8 hours, and spray granulate. The obtained powder is pretreated at 500° C. for 7 hours in an inert atmosphere to obtain carbon-coated nickel monoxide powder. Dissolve lithium acetate in 3.5L of anhydrous salt water, add carbon-coated nickel monoxide powder, stir evenly, ball mill for 3 hours, and spray granulate to obtain a dry powder. Put the powder into a rotary furnace, sinter at 650°C for 8h in N2 atmosphere, then heat up to 800°C for 5h, cool, sieve, classify by air flow and obtain the product.
[0019] The resulting product had a carbon content of 1.2%.
Embodiment 2
[0021] According to n(Li):n(Ni)=1.07, weigh 343g lithium hydroxide and 1000g nickel monoxide, add 150g organic carbon source sucrose according to the weight of nickel monoxide 15%, add nickel monoxide and sucrose 3.5L Mix evenly without salt water, ball mill for 8 hours, and spray granulate. The obtained powder is pretreated at 500° C. for 8 hours in an inert atmosphere to obtain carbon-coated nickel monoxide powder. Dissolve lithium hydroxide in 3.5L of anhydrous salt water, add carbon-coated nickel monoxide powder, stir evenly, ball mill for 3 hours, and spray granulate to obtain dry powder. Put the powder into a rotary furnace, sinter at 600°C for 8h in N2 atmosphere, then heat up to 800°C for 4h, cool, sieve, classify by air flow and obtain the product.
[0022] The resulting product had a carbon content of 1.6%.
Embodiment 3
[0024] According to n(Li):n(Ni)=1.05, weigh 336g of lithium hydroxide and 1000g of nickel monoxide, add 100g of organic carbon source polyethylene glycol according to the weight of 10% of nickel monoxide, and mix nickel monoxide and polyethylene glycol Diol was added and 3.0 L of anhydrous salt water were added, mixed evenly, ball milled for 3 hours, spray granulated, and the obtained powder was pretreated at 500° C. for 8 hours in an inert atmosphere to obtain carbon-coated nickel monoxide powder. Dissolve lithium hydroxide in 3.5L of anhydrous salt water, add carbon-coated nickel monoxide powder, stir evenly, ball mill for 3 hours, and spray granulate to obtain dry powder. Put the powder into a rotary furnace, sinter at 650°C for 8h in N2 atmosphere, then heat up to 820°C for 6h, cool, sieve, classify by air flow and obtain the product.
[0025] The resulting product had a carbon content of 2.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com