Mellitic acid rare-earth coordination polymer as well as preparation method and application
A technology of mellitic acid and complexes, which is applied in the field of preparation of rare earth metal coordination polymers, can solve the problems of weak absorption, low luminous efficiency, and weak luminescence of a single rare earth ion, and achieve high yield, good reproducibility, Effect of high thermal stability and phase purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Mellitic acid rare earth metal coordination polymer A Synthesis:
[0028] Dissolve mellitic acid (0.1 mmol, 34.2 mg) and dysprosium nitrate hexahydrate (0.3 mmol, 136.8 mg) in 10.0 mL of double distilled water, adjust the pH of the reaction mixture to 5 with solid sodium hydroxide, and stir the mixture for several Minutes later, it was sealed in a hydrothermal reaction kettle. After the reaction kettle was kept at 200°C for three days, it was heated at 5.0°C·h –1 The rate program cooled down to room temperature to obtain colorless blocky crystals, which were washed with water twice and dried in air.
Embodiment 2
[0030] Mellitic acid rare earth metal coordination polymer B Synthesis:
[0031] Dissolve mellitic acid (0.2 mmol, 68.4 mg) and erbium nitrate hexahydrate (0.5 mmol, 230.5 mg) in 12.0 mL of double-distilled water, adjust the pH of the reaction mixture to 4 with solid sodium hydroxide, and stir the mixture for several Minutes later, it was sealed in a hydrothermal reaction kettle. After the reaction kettle was kept at 180°C for three days, the –1 The rate program cooled down to room temperature to obtain colorless blocky crystals, which were washed with water twice and dried in air.
[0032] The mellitic acid rare earth metal coordination polymer prepared by the present invention (embodiment 1-2, A - B ) is characterized by the following structure:
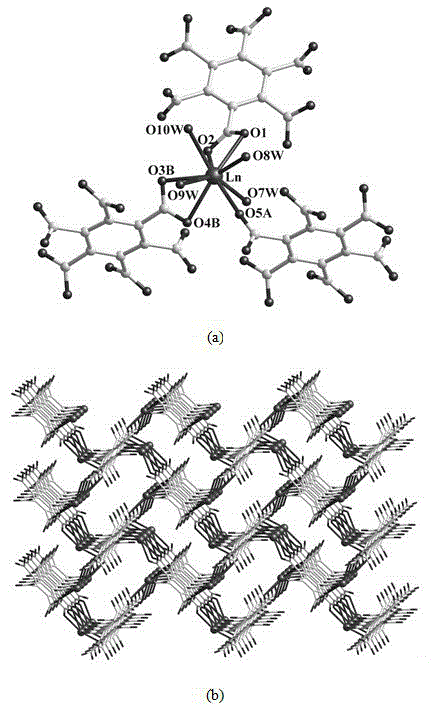
[0033] (1) Determination of crystal structure ( figure 1 )
[0034] Select a single crystal of a rare earth metal coordination polymer with a suitable size under a microscope, and use graphite monochromatized Mo-Kα rays (λ=...
Embodiment 3
[0042] Prepared by the present invention (embodiment 1-2, A - B ) Rare earth metal coordination polymer optical performance test is as follows:
[0043] After enriching the solid-state coordination polymer crystals prepared in Example 1-2, after further grinding, a solid fluorescence test was carried out on a JobinYvon (Horiba) Fluorolog-3 fluorescence spectrometer. The solid fluorescence test results are shown in Figure 4 . The test results show that under the excitation of 366nm ultraviolet light, the complex sample A has strong fluorescence emission peaks at 482, 573 and 600nm, and the luminescence intensity at 573nm is the largest; under the excitation of 371nm ultraviolet light, the complex sample B has There are two ligand-based fluorescence emission peaks at 417 and 434nm, in addition to a weaker fluorescence emission peak at 504nm.
[0044] The rare earth metal coordination polymer proposed by the present invention is a high-grade fluorescent material that can exis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com