Liposome delivery system for treating cartilage diseases and preparation method of liposome delivery system
A delivery system, liposome technology, applied in the field of medicine, can solve the problem that liposomes cannot be targeted into cartilage tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
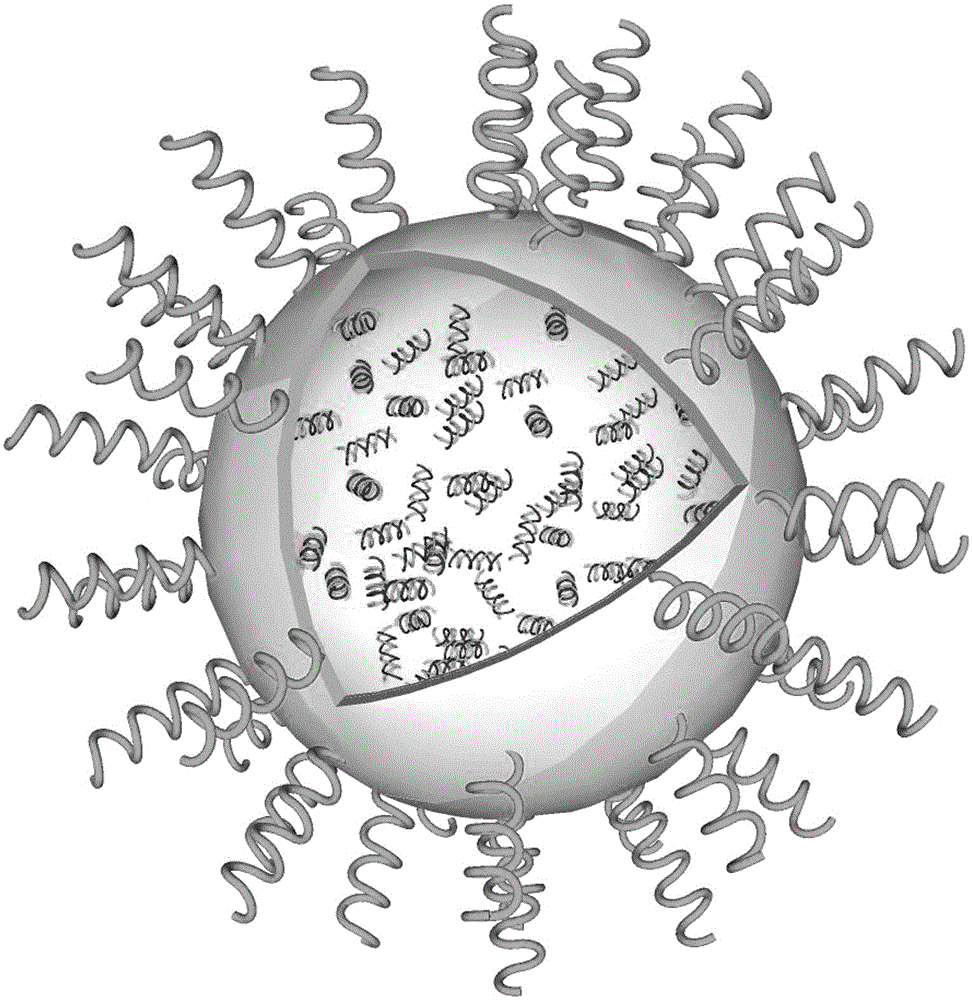
[0026] Embodiment 1: A liposome delivery system for the treatment of cartilage diseases, including liposomes, liposome modifications and small nucleic acid drugs, the liposomes are phosphatidylcholine DPPC, cholesterol, Dlin-KC2 -DMA; the liposome modification is polyethylene glycol lipid conjugate (C16PEG2000Ceramide) C-PEG, and the small nucleic acid drug is IhhsiRNA; its target sequence is SEQ ID NO: 2, which is the 1708-1734th part of IhhsiRNA Base sequence; the phosphatidylcholine DPPC concentration is 35mg / ml, accounting for 11.72% of the mass percent of liposomes and liposome modifications; the cholesterol concentration is 9.5mg / ml, accounting for liposomes and lipids The mass percentage of body modification is 23.85%; The concentration of polyethylene glycol lipid conjugate C-PEG is 50mg / ml, accounting for 16.74% of the mass percentage of liposome and liposome modification; Dlin-KC2-DMA The concentration is 28.5mg / ml, accounting for 47.69% of the mass percentage of lip...
experiment example 1
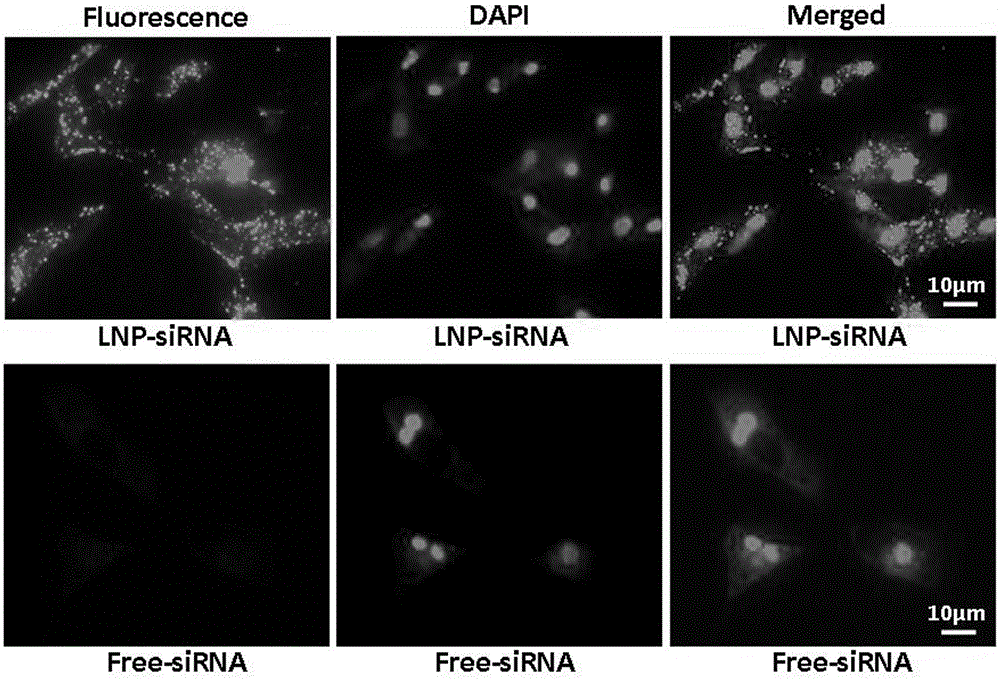
[0034] Experimental example 1: Verification of liposome delivery system into chondrocytes
[0035] 1. Liposome delivery of siRNA into chicken hypertrophic chondrocytes
[0036] Experimental materials: 1 / 3 chondrocytes of 17-day chicken embryo sternum, lipid nanoparticle (LNP), negative control siRNA (NegativeControlsiRNA). 0.3% EDTA-free trypsin, 0.9% collagenase, 0.3% hyaluronidase [Type I, Sigma, Cat. No. H3506-1G].
[0037] Experimental method: The LNP-siRNA complex was prepared as described in Example 1. Obtain chondrocytes from 1 / 3 of the sternum of 15 17-day-old chicken embryos, cut them into pieces, put them in a 50ml centrifuge tube, and then add 1ml of each of the three digestive solutions, 3ml in total (digestive solution preparation: 0.3% EDTA-free pancreatic Protease, 0.9% collagenase, 0.3% hyaluronidase (prepared by volume 1:1:1), placed in a 37°C water bath for 30 minutes, carefully sucked up the upper liquid, and then added 1ml each of the three digestive solu...
experiment example 2
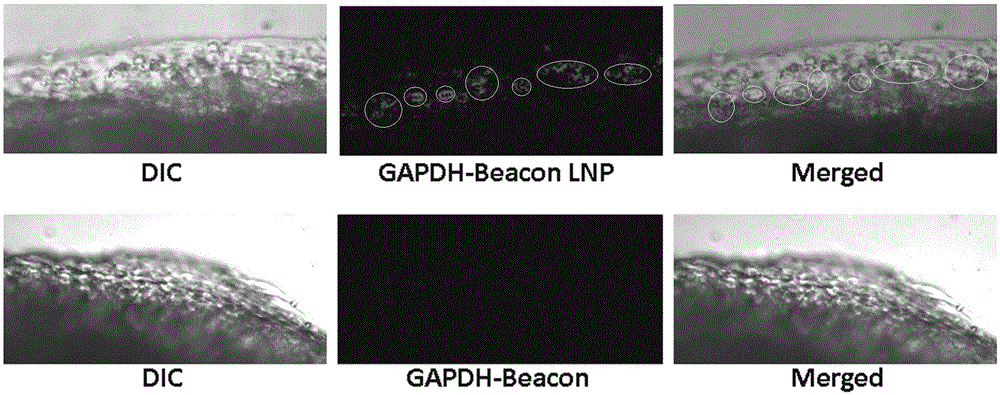
[0046] Experimental Example 2: Verification of the expression of IhhmRNA in rats after the prepared LNP-siRNA complex was systematically treated:
[0047] Experimental materials: 10 Wistar rats. Lipid nanoparticle (LNP), Ihh.siRNA solution: take 10ul of Ihh.siRNA with a concentration of 20uM, dilute it to 40ul for use.
[0048] Experimental method: The LNP-Ihh.siRNA complex was prepared as described in Example 1. 40ul of the LNP-Ihh.siRNA complex was injected into the right knee joint of the rat, and 40ul of the Ihh.siRNA solution was injected into the left knee joint. After 48 hours, the rats were sacrificed, the whole knee articular cartilage of the rats was scraped, and the total RNA was extracted, reverse-transcribed into cDNA, and detected by Real-time PCR.
[0049] Ihh mRNA primer: sense strand: 5'- CAGGAAGGACCCATTCCGTC- 3' ;antisense strand: 5'- AAGTCACAAACCCAGGTCCC- 3' .
[0050] The results showed that the expression of IhhmRNA decreased by 80% in the rat right k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com