Production of sulfuric acid from coke oven gas desulfurization product
A technology for coke oven gas and sulfuric acid, which is applied to the preparation/purification of sulfur, separation methods, sulfur compounds, etc., can solve the problems of increased corrosiveness of sulfuric acid products, and achieve the effects of simple process equipment, reduced impact, and cheap process equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
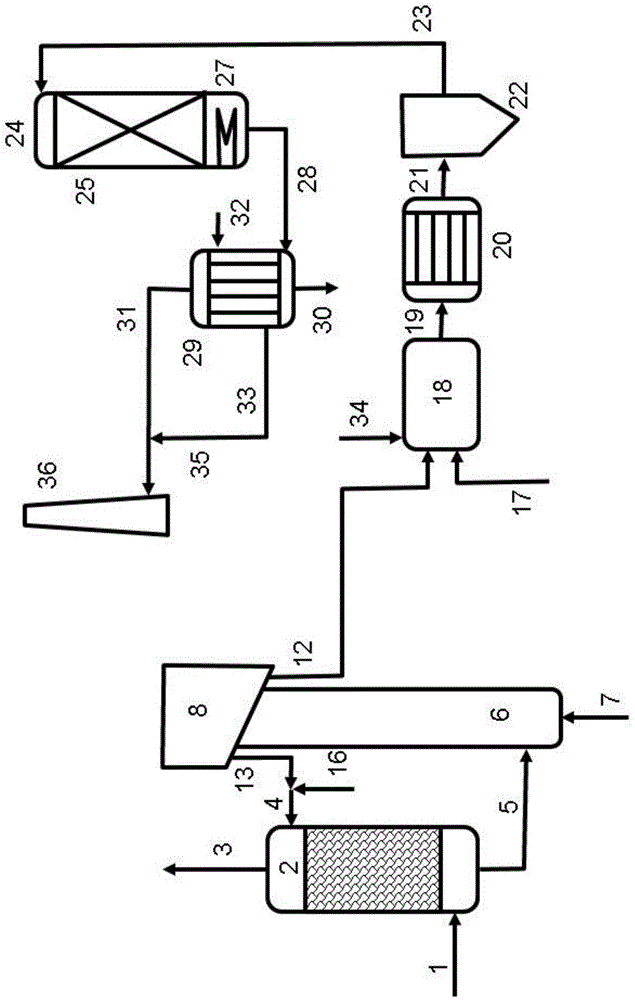
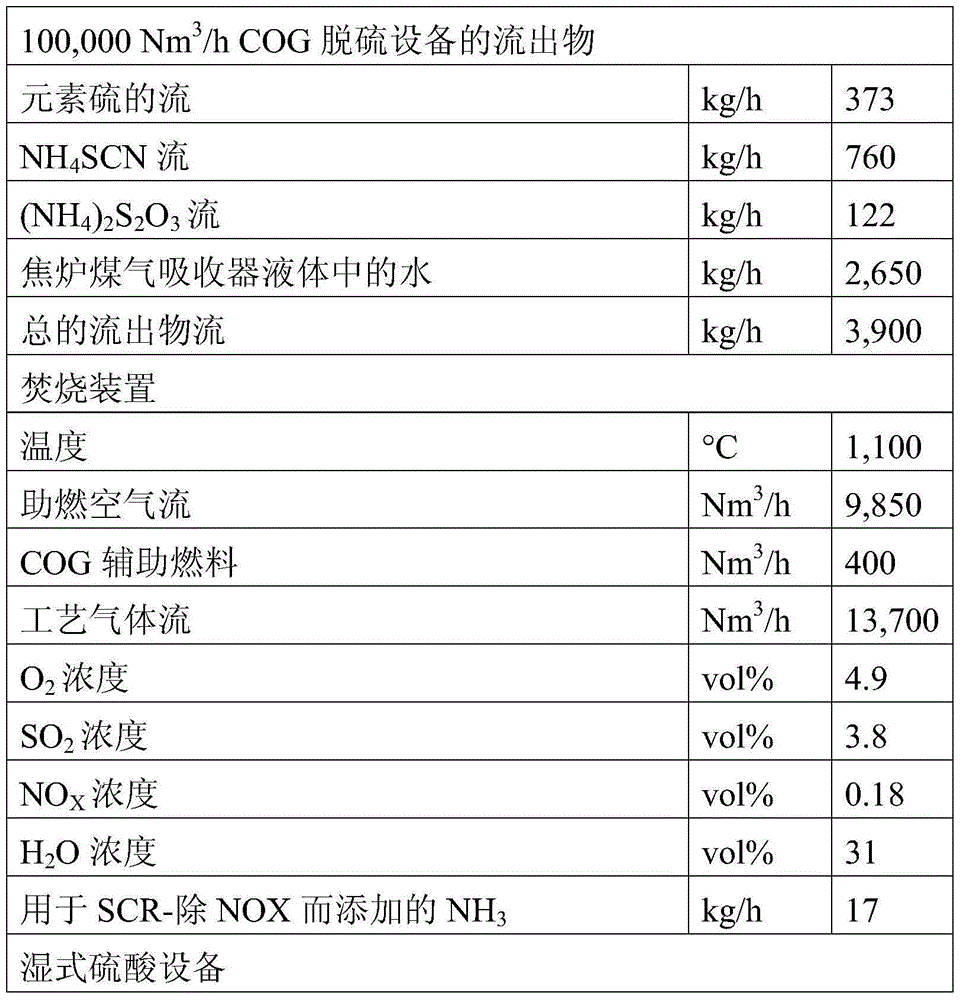
[0070] In an embodiment according to the present disclosure, using such as figure 2 The method shown, which involves ammonia absorbent solution and quinone type H 2 S oxidation catalyst. Key parameters used to treat typical coke oven gas (given in Table 1) in a wet sulfuric acid process are shown in Table 2. The overall process includes coke oven gas desulfurization, incineration of sulfur sludge and production of coke oven gas absorber liquid and sulfuric acid in a wet sulfuric acid plant.
[0071] The coke oven gas is cooled to about 30°C and contacted with a liquid ammonia absorbent solution which also contains dissolved quinone catalyst. The alkaline absorber according to this embodiment operates at approximately atmospheric pressure. In a reactor operating at about 30°C, about 50% of the H 2 S is converted to elemental sulfur, forming a liquid slurry. remaining H 2 S is further oxidized to a large number of ions containing sulfur and oxygen, which for simplicity ar...
Embodiment 2
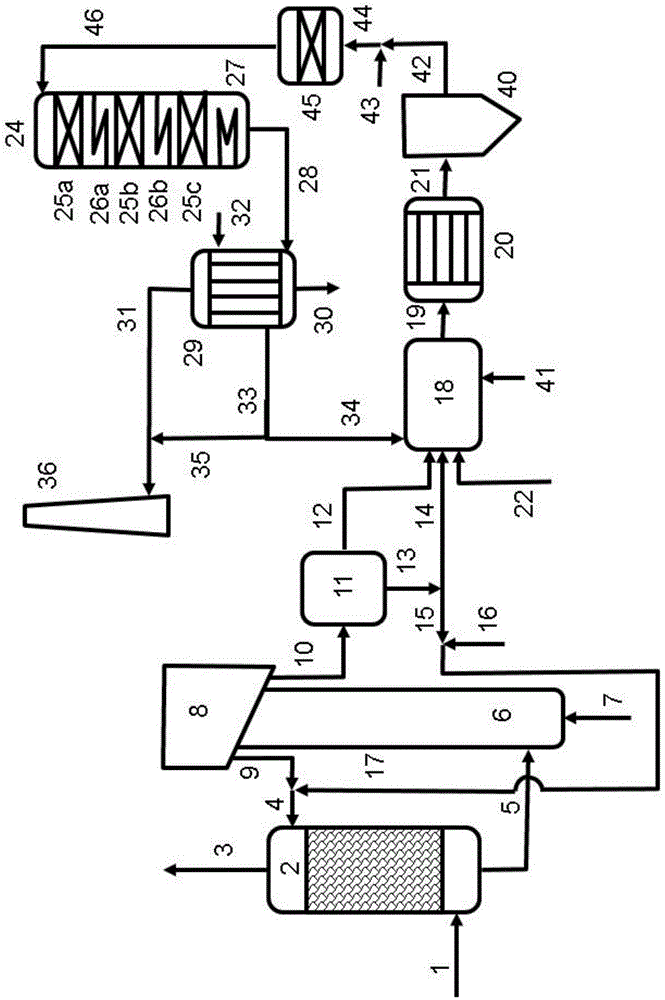
[0086]Example 2 involves a process operated under similar conditions to Example 1, but with an alternative nitrogen removal system.
[0087] The process gas leaving the incinerator is similar to that in Example 1, except that it is now assumed that the content of N from the feed (SCN - and NH 4 + -salt) to form 1.5% NO X .
[0088] uncontrolled NO X The concentration will be 540ppm by volume, equivalent to 1,230mg / Nm 3 (in NO 2 calculate). Thanks to SO 2 The oxidation catalyst also oxidizes part of the colorless NO into red NO 2 , so the process gas leaving the chimney will have a distinct color.
[0089] if NO 2 Concentrations can be lower than about 280mgNO 2 / Nm 3 , the NO formed during incineration X Keep below about 1% N in the feed, then the chimney plume can be colorless without the need for a nitrogen removal process, but the NO X Concentrations may still be above local discharge limits.
[0090] The temperatures in the incinerator and in the combustion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com