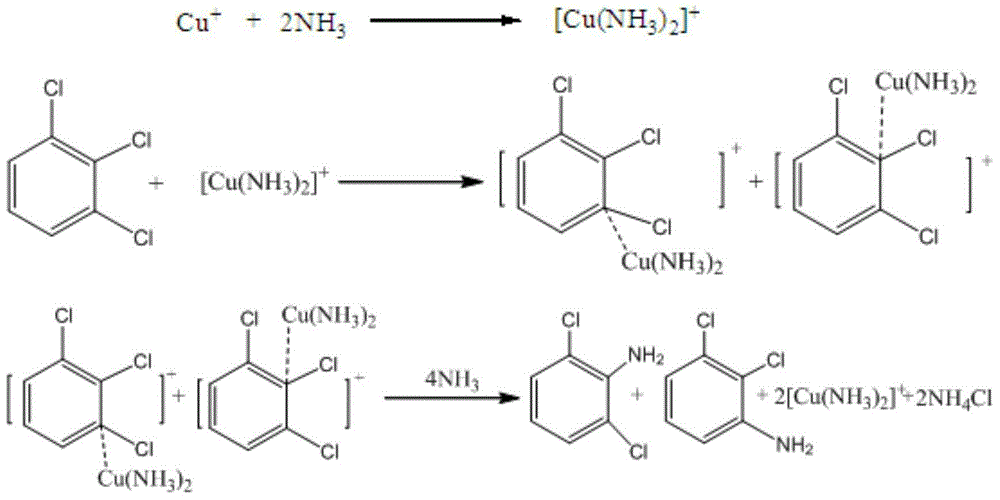
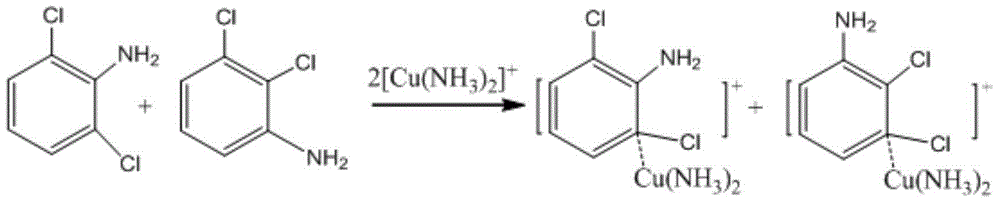
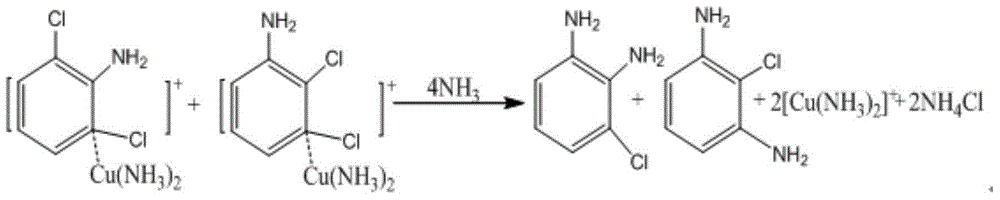
Method for preparing 2,3-/2,6-dichloroaniline
A technology of dichloroaniline and trichlorobenzene, which is applied in the field of preparation of 2,3-/2,6-dichloroaniline, can solve the problems of cumbersome preparation and purification routes, high cost of raw materials, and high requirements for equipment materials, so as to reduce separation Process difficulty, reduced production cost, and high atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Throw 280.4g (6.60mol) of 40% ammonia water into a 1L autoclave, 2.8g (content 99.0%, 0.016mol) of copper chloride dihydrate, 100.0g (content 99.8%, 0.55mol) of 1,2,3-trichlorobenzene , the temperature was raised to 180°C, the pressure was 45kgf, the stirring speed was 600rpm, and the heat preservation reaction was carried out for 30 hours. After the reaction was completed, the temperature was lowered to about 80°C. 20% NaOH solution was used to adjust the pH value to 12, and Cat was recovered by filtration, and the filtrate was separated to obtain 99.2 g of ammonolysis oil layer, quantitative 1,2,3-TCB77.20%, 2,3-DCA13.36%, 2,6-DCA6. 44%, diaminochlorobenzene 0.98%. The experimental data are shown in Table 1.
[0025] Table 1 Effect of different ammonia concentrations on 1,2,3-TCB ammonolysis
[0026]
Embodiment 2
[0028] Other same example 1, 40% ammonia water feeding amount 373.9g (8.80mol), the pressure in the process is 57kgf, after the reaction is completed, the pressure is released, the base is adjusted and filtered, and the ammonolysis oil layer is obtained by layering 98.7g, quantitatively 1,2,3-TCB75.39 %, 2,3-DCA13.61%, 2,6-DCA6.98%, diaminochlorobenzene 0.98%. The experimental data are shown in Table 2.
[0029] The influence of the molar ratio of table 2 ammonia water and 1,2,3-TCB on ammonolysis
[0030]
Embodiment 3
[0032] Others are the same as Example 1, ammonolysis catalyst throws 1.68g of cuprous chloride (content 97%, 0.016mol), after the reaction is completed, the temperature is lowered and the pressure is released, filtered, and stratified to obtain 97.7g of ammonolysis oil layer, quantitatively 1,2,3-TCB72. 34%, 2,3-DCA17.08%, 2,6-DCA8.36%, diaminochlorobenzene 0.96%. The experimental data are shown in Table 3.
[0033] Table 3 Effects of different catalysts on the ammonolysis of 1,2,3-TCB
[0034]
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com