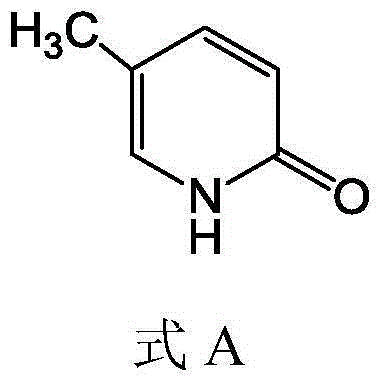
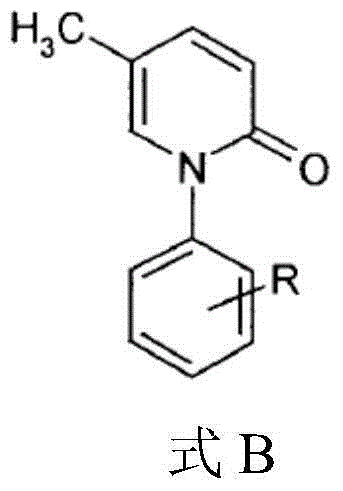
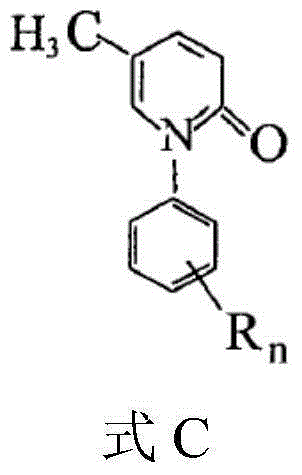
5-methyl-2(1H)pyridone derivatives, and preparation method and application thereof
A technology for pyridone and derivatives, which is applied in the field of 5-methyl-2-pyridone derivatives and its preparation, and can solve the problems of preparation methods and uses of 5-methyl-2(1H) pyridone derivatives, There are no other problems, and the effect of low cost, simple steps and few processes is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]
[0079] Among them, "rf" is the abbreviation of reflux, and its Chinese meaning is "reflux".
[0080] In a 25ml reaction bottle, first add 3.4ml from 17mlH 2 O and the solution (50%, volume fraction) that 17ml concentrated sulfuric acid forms, then add 1g (0.01mol) 2-amino-5-picoline, cool to below 10 ℃ with ice-salt bath, after stirring several minutes, reaction solution Turned milky white. Then slowly drop by (1.72gNaNO 2 with 3mlH 2O) the mixed solution, during dropwise addition, produces irritating gas, after dropwise addition, the reaction solution turns into a light yellow solution, and TCL (thin layer chromatography) monitors until the reaction is complete (about 40min). Then add 8mlH 2 O, reflux stirring reaction 15min, cooling, add anhydrous Na under stirring 2 CO 3 , make the reaction solution neutral (produce yellow-brown solid), filter, the gained filtrate is spin-dried, then dissolve and filter with absolute ethanol, and the gained filtrate is spi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com