Poly(ethylene 2,5-furandicarboxylate) with low diethylene glycol link content and preparation method of poly(ethylene 2,5-furandicarboxylate)
A technology of polyethylene furandicarboxylate and ethylene furandicarboxylate chain links, applied in the field of bio-based polyester-polyethylene furandicarboxylate and its preparation, can solve the problem of slow crystallization of PEF, ether Chemical side reactions, affecting crystallinity and other problems, to achieve the effect of increasing molecular weight, high selectivity, and excellent crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and stannous oxalate (0.1mmol, 20.7mg) into a 250mL three-necked flask, the alkyd molar ratio was 2.5, and reacted at 150-200°C for 2 hours to obtain an esterified product with an esterification rate of 98.7%;
[0052] (2) Add ethylene glycol antimony (0.1mmol, 42.4mg), antioxidant 1010 (90mg), antioxidant 168 (90mg) to the esterification product obtained in step (1), at 200-220°C, 10 5 Under the condition of -2000Pa, the precondensation reaction was carried out for 0.5 hours to remove free ethylene glycol and a small amount of oligomers to obtain a prepolymer;
[0053] (3) The precondensation product obtained in step (2) was subjected to melt polycondensation reaction at 240-250° C. and about 300 Pa for 2 hours to obtain polyethylene furandicarboxylate PEF1.
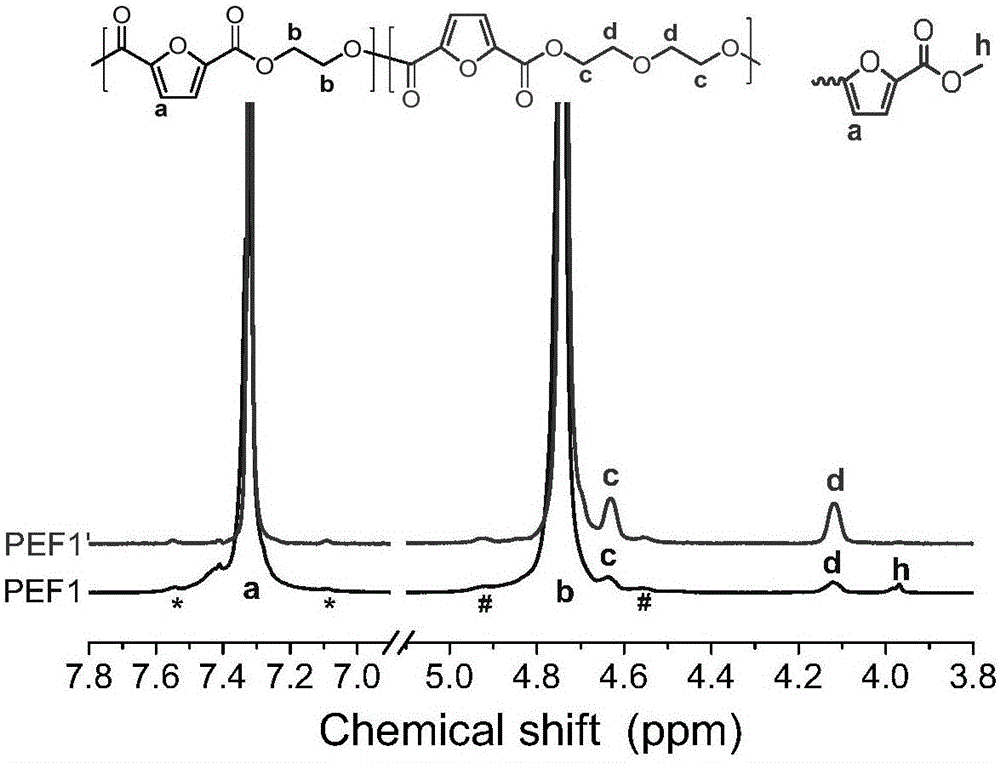
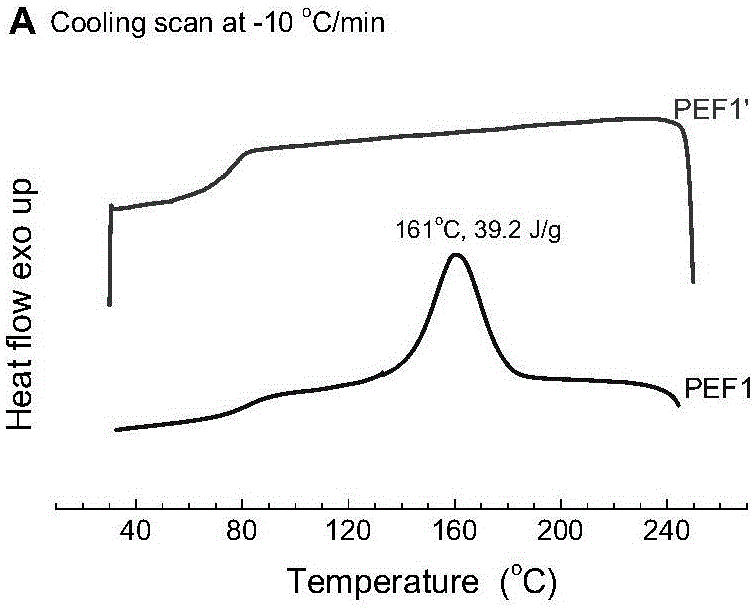
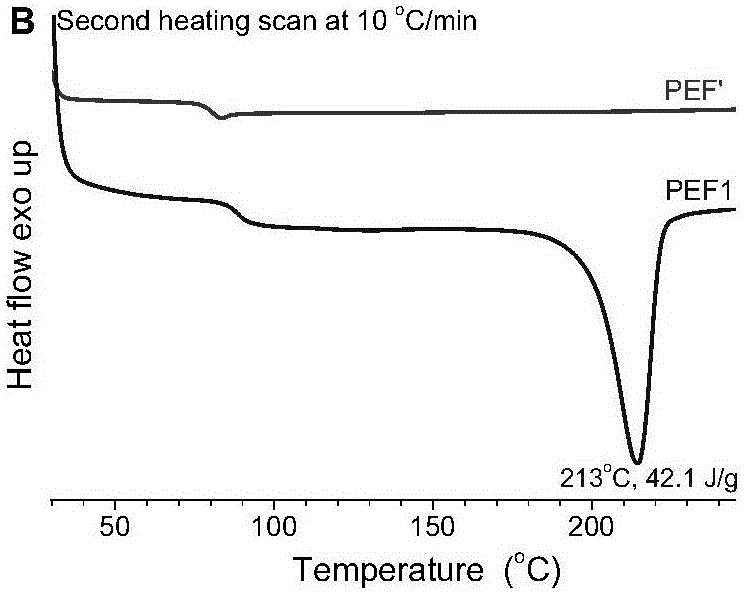
[0054] The diethylene glycol chain segment content of PEF1 is 1.76mol%, the intrinsic viscosity is 0.62dl / g; the melt crystal...
Embodiment 2
[0056] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and titanium glycolate (0.1mmol, 16.8mg) into a 250mL three-necked flask, The ratio is 2.5, and the esterification product is obtained at 150-200°C for 2 hours, and the esterification rate reaches 98.7%;
[0057] (2) Add ethylene glycol antimony (0.1 mmol, 42.4 mg), triphenyl phosphite (90 mg), and phosphorous acid (50 mg) to the esterification product obtained in step (1), and heat 5 Under the condition of -2000Pa, the precondensation reaction was carried out for 0.5 hours to remove free ethylene glycol and a small amount of oligomers to obtain a prepolymer;
[0058] (3) The precondensation product obtained in step (2) was subjected to melt polycondensation reaction at 240-250° C. and about 300 Pa for 2 hours to obtain polyethylene furandicarboxylate PEF2.
[0059] The diethylene glycol chain segment content of PEF2 is 1.96mol%, and the intrinsic viscosity is 0.63dl / g; the melt cryst...
Embodiment 3
[0061] (1) Add dimethyl furandicarboxylate (0.1mol, 18.4g), ethylene glycol (0.25mol, 15.5g) and n-butyl titanate (0.05mmol, 17mg) into a 250mL three-necked flask, alkyd mole Ratio of 2.5, reacted at 150-200°C for 4 hours to obtain an esterified product with an esterification rate of 98.3%;
[0062] (2) Add 1,8-diazabicycloundec-7-ene (0.1mmol, 15.2mg), antioxidant 1010 (90mg), antioxidant 168 (90mg), at 200-220°C, 10 5 Under the condition of -2500Pa, the precondensation reaction was carried out for 0.5 hours, and the free ethylene glycol and a small amount of oligomers were removed to obtain the prepolymer;
[0063] (3) The precondensation product obtained in step (2) was subjected to melt polycondensation reaction at 240-250° C. and about 200 Pa for 4 hours to obtain polyethylene furandicarboxylate PEF3.
[0064] The diethylene glycol chain segment content of PEF3 is 0.53mol%, the intrinsic viscosity is 0.49dl / g; the melt crystallization peak temperature is 157°C, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Enthalpy of crystallization | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com