Method for recycling tungsten ions from scheelite waste mineral processing wastewater
A technology for beneficiation wastewater and scheelite, applied in mining wastewater treatment, chemical instruments and methods, tungsten compounds, etc., can solve the problems of small adsorption amount of tungsten ions, low practical value, low elution rate, etc., and achieve recovery rate. High, easy to operate, large environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
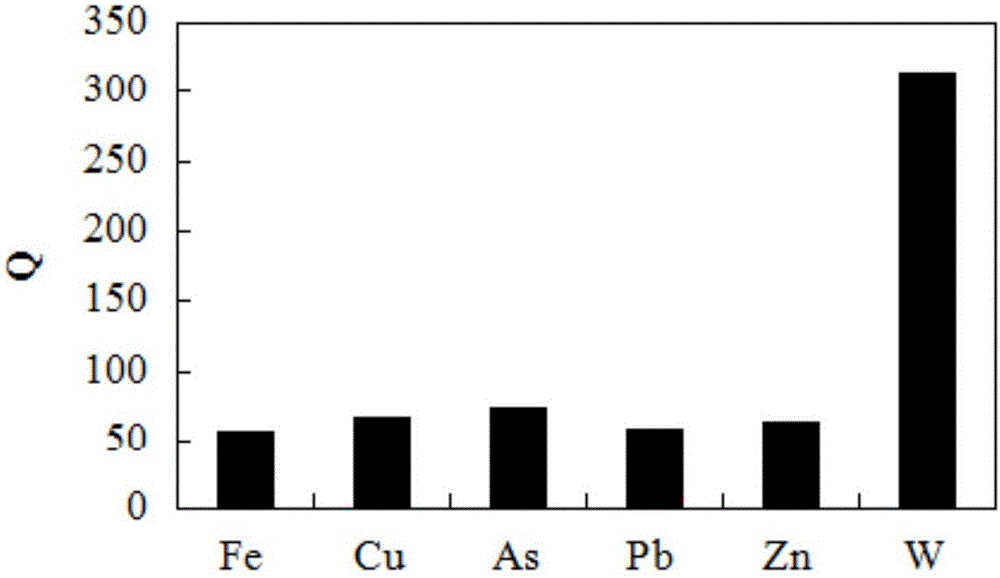
[0025] 1), choose to use chlorine spheres as a parent (chlorine spheres are macroporous chloromethylated cross-linked polystyrene microspheres), and morpholine (MPL) is a ligand to prepare a heterocyclic chelating resin containing O and N as Adsorbent for heavy metal tungsten ions.
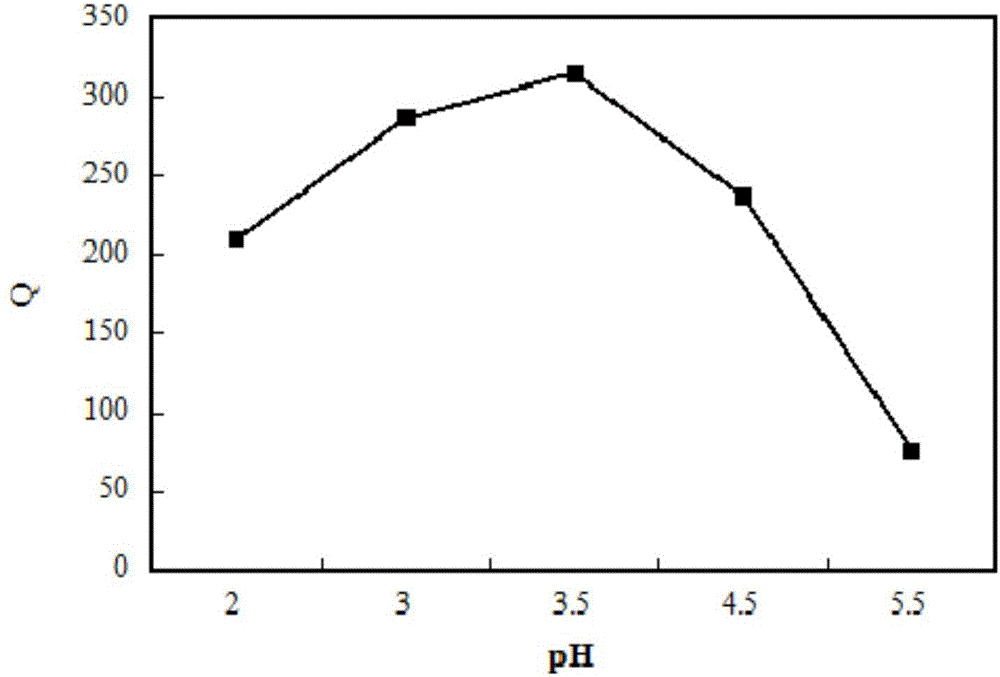
[0026] 2) Accurately weigh 14 mg of chelating resin containing O, N heterocycles into a 100 mL iodine flask, add 28 mL of acetic acid-sodium acetate buffer solution with pH=3.5, and soak for 24 hours.
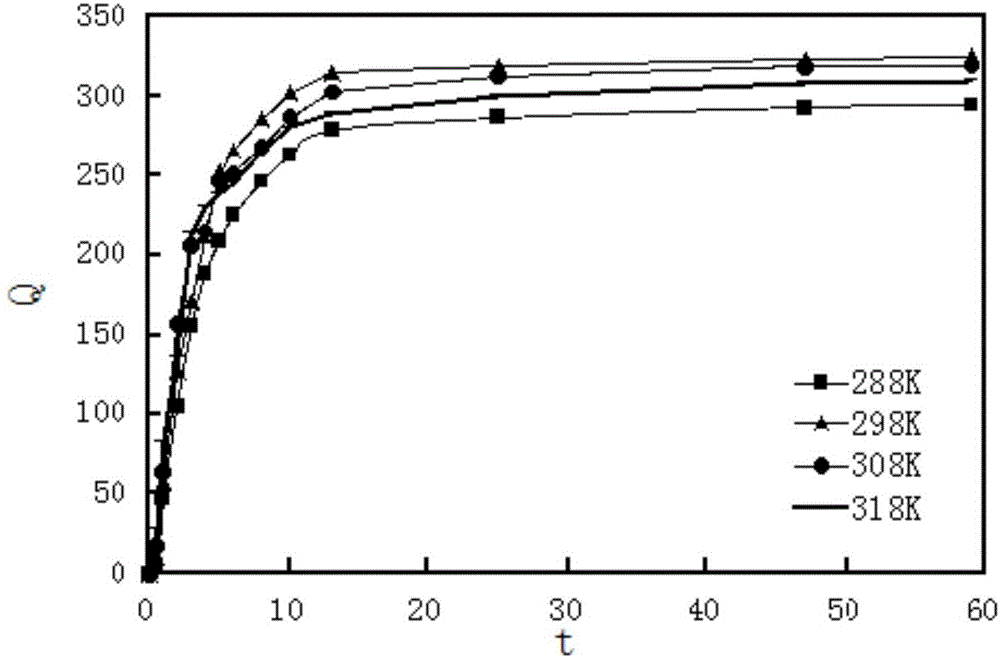
[0027] 3) Add 2 mL of scheelite mineral processing wastewater solution containing 2.5 mg / mL tungsten ions, and shake at T=298K at a constant temperature of r=100 r / min until adsorption equilibrium.
[0028] 4) After the adsorption equilibrium, measure the saturated adsorption capacity of the chelating resin containing O, N heterocyclic rings for tungsten ions.
[0029] 5), the chelating resin containing O and N heterocyclic rings after adsorption equilibrium is washed with an acetic acid-sodium ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com