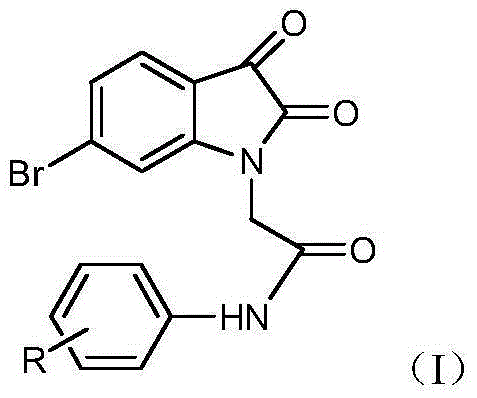
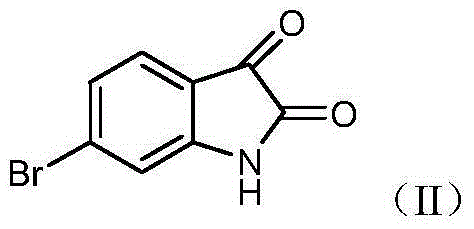
Isatin derivative 6-bromo-2,3-dioxoindolin-N-halogen substituted phenylacetamide, and preparation method and application thereof
A technology of dioxoindoline and phenylacetamide, applied in the application of antiepileptic drugs, in the field of preparation of antidepressants, which can solve problems such as depression, aggravation of depression, and reduction of folic acid levels, and achieve rapid onset and short reaction time Flexible, low neurotoxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 16
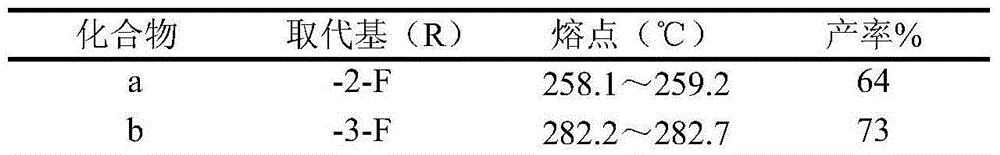
[0023] Embodiment 16-Bromo-2,3-dioxoindoline-N-halogen substituted phenylacetamide derivative physical constant
[0024]
[0025]
[0026] (a) Preparation of 6-bromo-dioxoindoline-1-yl-N-(2-fluorophenyl)acetamide
[0027] Add 30mL DMF to a 50mL round-bottomed flask, add 3.4mmol compound II (6-bromo-2,3-indoledione) 0.5g in batches under ice-cooling, stir and dissolve, then add 3.4mmol K 2 CO 3 0.3g, stirred at room temperature for 1h. Add 0.42 g of 3.4 mmol of 2-fluorophenyl chloroacetamide and 0.5 g of potassium iodide 6 mmol, raise the temperature to 80° C. for 8 h, and monitor the reaction by TLC until the reaction is complete. Cool to room temperature, add distilled water about 6 times the volume of the reaction solution, add dropwise dilute hydrochloric acid to adjust the pH value of the solution to 3-4, stir for 10 min, filter with suction, dry, and recrystallize the crude product with ethanol to obtain product a. The spectral data of the compound are as follows...
Embodiment 2
[0038] Embodiment 2 anticonvulsant pharmacological experiment and neurotoxicity experiment
[0039] The forced swimming test is the most commonly used experiment for the study of depressants and antidepressants. The sum of the time for mice to give up swimming and struggling in the water is called the immobility time, which reflects the symptoms of helplessness in mice, while the forced swimming test The prolongation of the immobility time in the middle showed depression-like symptoms. The compound provided by the invention is screened by the mouse forced swimming test to evaluate its antidepressant efficacy and safety.
[0040] (1) Mice forced swimming test:
[0041] a method: see (PorsoltRDetal, ArchIntPharmaeodynTher, 1997) and other reported methods, after intraperitoneal injection for 30 minutes, put the mouse into the XSC-mouse constant temperature swimming instrument (in a bucket with a water depth of 10cm and a diameter of 34cm, the water temperature is 25 ± 2°C), ob...
Embodiment 3
[0051] Convulsion experiment induced by embodiment 3 pentylenetetrazole
[0052] The invention provides a basis for studying the therapeutic effect of the isatin derivative 6-bromo-2,3-dioxoindoline-N-halogen substituted phenylacetamide on a mouse convulsion model induced by pentylenetetrazole.
[0053] a Model establishment: The convulsion mouse model was injected intraperitoneally with 90 mg / kg pentylenetetrazole, and the occurrence of convulsions in mice was observed within 30 minutes after intraperitoneal injection of pentylenetetrazole. Convulsions were diagnosed according to the Racine standard, and the acute convulsion model was successfully established if the mice had seizures of grade 4 or above.
[0054] b Observation indicators: observe the number of mice with convulsions in each group, calculate the percentage of convulsions, compare the difference between the administration group and the control group, and determine whether there is anticonvulsant activity. Index...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com