Glucosamine-containing 1,3,4-thiadiazole derivative and its preparation method and use
A technology of thiadiazole derivatives and glucose, which is applied in the field of drug preparation and can solve problems such as low bioavailability, hepatotoxicity, and narrow therapeutic range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
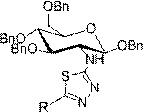
[0026] Embodiment 1, a kind of glucosamine-containing 1,3,4-thiadiazole derivatives, its molecular formula is as follows:
[0027]
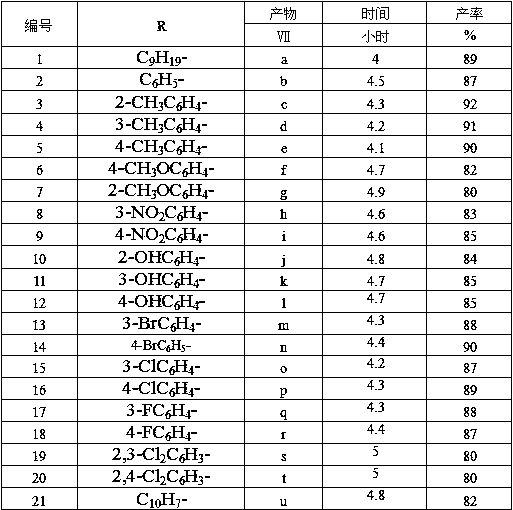
[0028] Wherein, the R is selected from C 9 h 19 -,C 6 h 5 -, 2-CH 3 C 6 h 4 -, 3-CH 3 C 6 h 4 -, 4-CH 3 C 6 h 4 -,4-CH 3 OC 6 h 4 -,2-CH 3 OC 6 h 4 -,3-NO 2 C 6 h 4 -, 4-NO 2 C 6 h 4 -,2-OHC 6 h 4 -, 3-OHC 6 h 4 -, 4-OHC 6 h 4 -,3-BrC 6 h 4 -, 4-BrC 6 h 4 -, 3-ClC 6 h 4 -,4-ClC 6 h 4 -, 3-FC 6 h 4 -,4-FC 6 h 4 -,2,3-Cl 2 C 6 h 3 -,2,4-Cl 2 C 6 h 3 -,C 10 h 7 -.
Embodiment 2
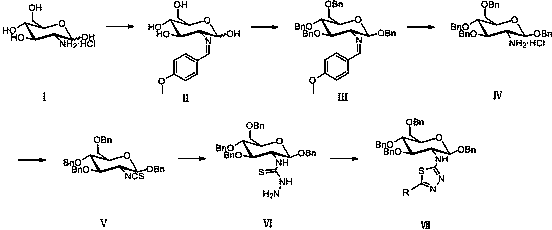
[0029] Embodiment 2, a kind of synthetic method containing glucosamine 1,3,4-thiadiazole derivative as described in embodiment 1, its steps are as follows:
[0030] (1) Preparation of 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl-β-D-glucopyranose: 2-deoxy-2-amino-1 ,3,4,6-Tetra-O-benzyl- β -D-pyranose hydrochloride, carbon disulfide and triethylamine are reacted, and then reacted with p-toluenesulfonyl chloride to obtain 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O- Benzyl- β -D-glucopyranose; reaction with acetonitrile as solvent, 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β - The molar ratio of D-pyranose hydrochloride to carbon disulfide, triethylamine and p-toluenesulfonyl chloride is 1:1:3:1, the reaction temperature is 0°C, and the reaction time is 1 hour.
[0031] (2) N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β -D-glucopyranose-2-yl)-N'-thiosemicarbazide preparation: 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl-β- The reaction of D-glucopyranose with hydrazine hydrate give...
Embodiment 3
[0033] Embodiment 3, a kind of synthetic method containing glucosamine 1,3,4-thiadiazole derivative as described in embodiment 1, its steps are as follows:
[0034] (1) Preparation of 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl-β-D-glucopyranose: 2-deoxy-2-amino-1 ,3,4,6-Tetra-O-benzyl- β -D-pyranose hydrochloride, carbon disulfide and triethylamine are reacted, and then reacted with p-toluenesulfonyl chloride to obtain 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O- Benzyl- β -D-glucopyranose; reaction with acetonitrile as solvent, 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β - The molar ratio of D-pyranose hydrochloride to carbon disulfide, triethylamine and p-toluenesulfonyl chloride is 1:1.2:3.5:1.2, the reaction temperature is 0°C, and the reaction time is 2 hours.
[0035] (2) N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β -D-glucopyranose-2-yl)-N'-thiosemicarbazide preparation: 2-deoxy-2-isothiocyanate-1,3,4,6-tetra-O-benzyl-β- The reaction of D-glucopyranose with hydrazine hydra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com