Novel CT contrast medium and preparation method and application thereof
A technology of contrast agents and nanoparticles, which is applied in the preparation of X-ray contrast agents, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve the problems of low X-ray attenuation coefficient, heavy heart load, enhanced scanning, etc. Good compatibility and excellent contrast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
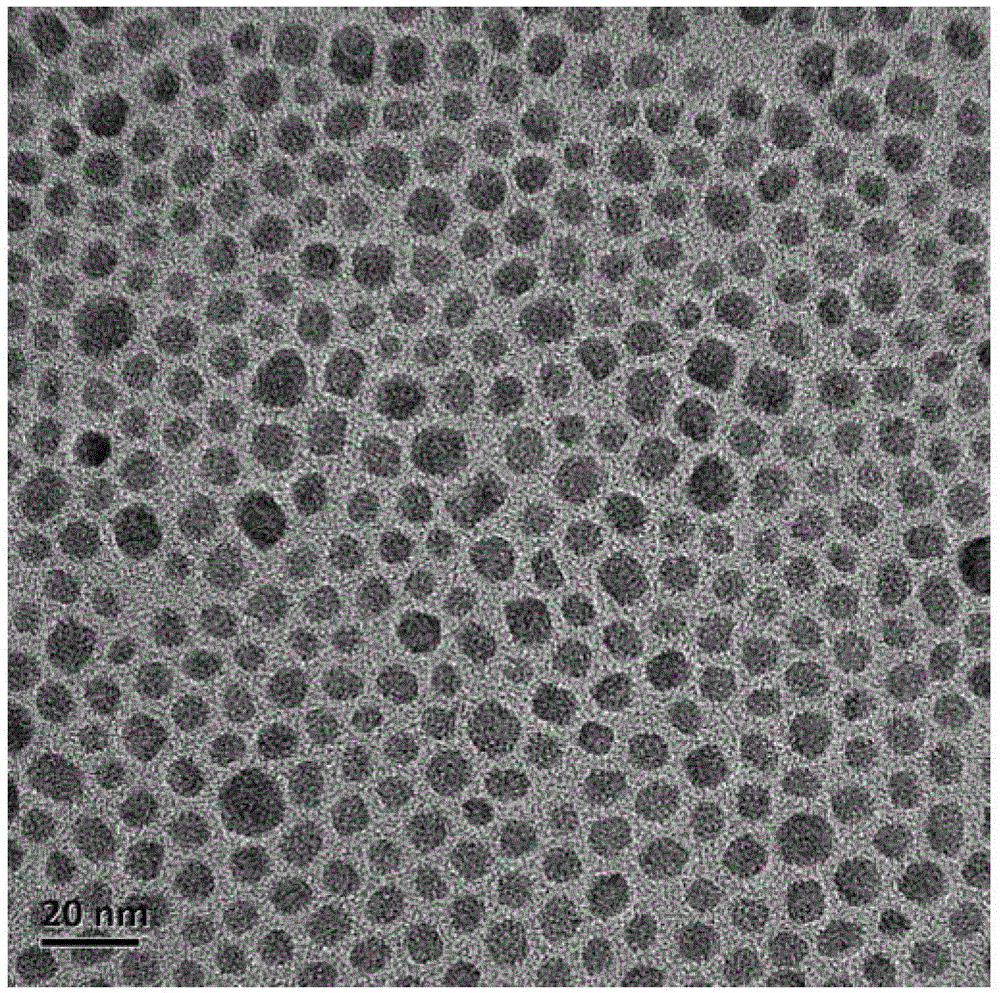
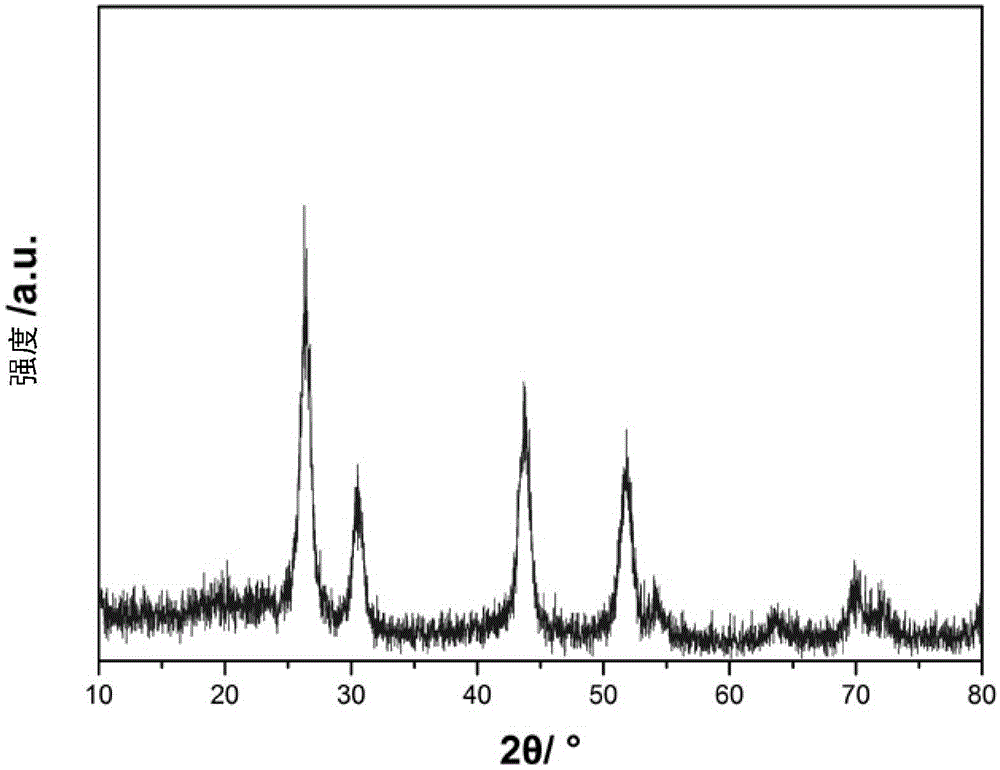
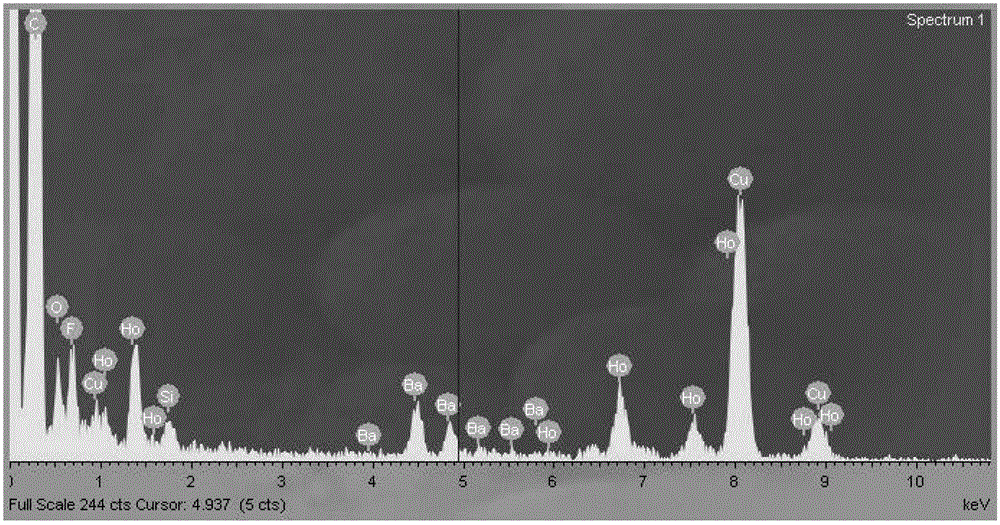
[0059] Weigh 1mmolHoCl respectively 3 ·6H 2 O (379.38 mg) and BaCl 2 2H 2 O (244.26mg), dissolved in 5mL of deionized water for later use; add 20mL of absolute ethanol, 12mL of oleic acid and 12mL of oleylamine to the lining of the hydrothermal kettle, stir magnetically for 0.5 hours, and then add the pre-prepared The above-mentioned chloride aqueous solution containing rare earth ions was stirred at room temperature for 1 hour; then 5 mL of aqueous solution containing 210 mg of NaF was added dropwise, stirred at room temperature for 1 hour, and then the hydrothermal kettle was placed in a constant temperature box at 160 ° C for 12 hours of hydrothermal reaction. When it was a child, take it out and cool it down to room temperature naturally, and then carry out centrifugation; the collected solids are cleaned successively with cyclohexane and ethanol for 3 times; 5 particles);
[0060] Take 3 mL of the above chloroform solution, add 1 ml of phospholipid PEG chloroform solu...
Embodiment 2
[0069] Medical Imaging Application Effect Experiment
[0070] 1. CT imaging
[0071] 1.1 Experimental materials and instruments:
[0072] The nCAs hydrophilic particles prepared in Example 1;
[0073] CT imaging detection instrument model: PhilipsBrillianceiCT256; PhilipsMedicalSystems, Cleveland, OH
[0074] 1.2 Experimental animals: healthy male Sprague-Dawley rats, with an average age of 2 months and an average weight of 200 g, purchased from the animal room of Fudan University School of Medicine;
[0075] 1.3 Experimental method: After SD rats were intraperitoneally anesthetized with chloral hydrate, the nCAs aqueous solution and clinical iohexol solution (both 52 mg / mL, 260 mg / kg) prepared in Example 1 were catheterized through the rat femoral vein Inject boluses into normal rats, perform CTA imaging, and observe the changes in the CT values of the heart, liver, spleen, kidney, and bladder at different time points; in addition, to prepare a stroke model in the left m...
Embodiment 3
[0081] Toxicity evaluation experiment
[0082] 1. In vitro cytotoxicity test
[0083] 1.1 Experimental materials:
[0084] The nCAs hydrophilic nanoparticles prepared in Example 1;
[0085] 1.2 Experimental method:
[0086]MTT (3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazoliumbromide) method was used to evaluate cell viability, and the specific experimental methods were: (1) inoculated cells: cultured with 10% fetal calf serum solution to make a single cell suspension, 10 per well 5 -10 6 Cells were inoculated into a 96-well plate, with a volume of 100 microliters per well. (2) Cell culture: After adding nanoparticles and co-cultivating with cells for 24 and 48 hours, add MTT solution (5mg / ml, prepared with PBS, pH = 7.4) 50 microliters, continue to co-cultivate for 4 hours, carefully aspirate and discard the culture supernatant in the well, for suspension cells, centrifuge and then aspirate and discard the culture supernatant in the well. (3) Quantification: add 150 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com