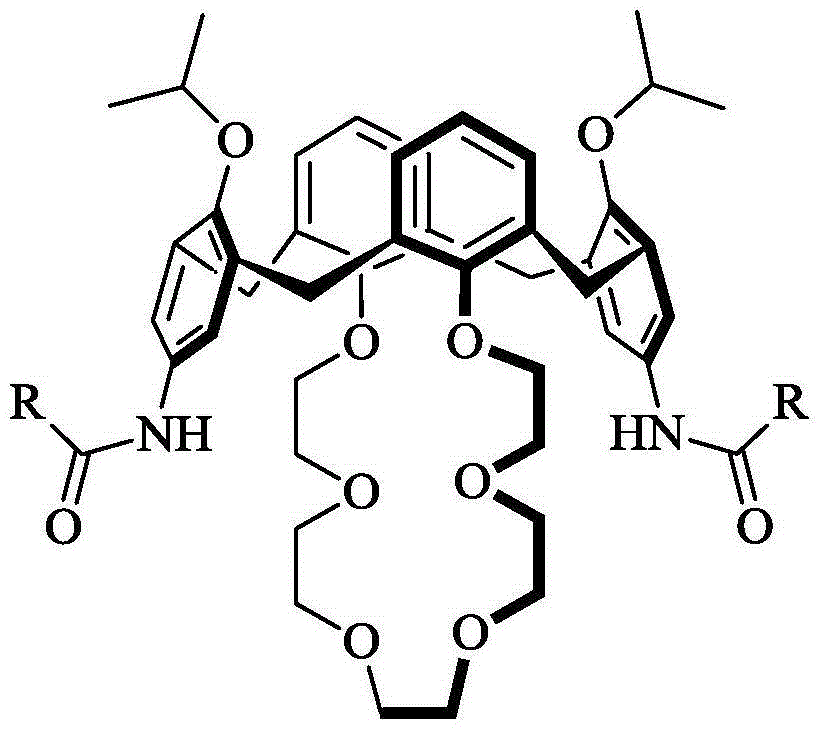
Bisamide-substituted novel calixcrown ether compound as well as synthesis method and application thereof
A bisamide and hydrocarbon crown ether technology is applied in the field of organic extractants, which can solve the problems of low distribution ratio, poor fat solubility, low cesium distribution, etc., and achieves the effects of high reaction efficiency, simple purification process and improved extraction performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
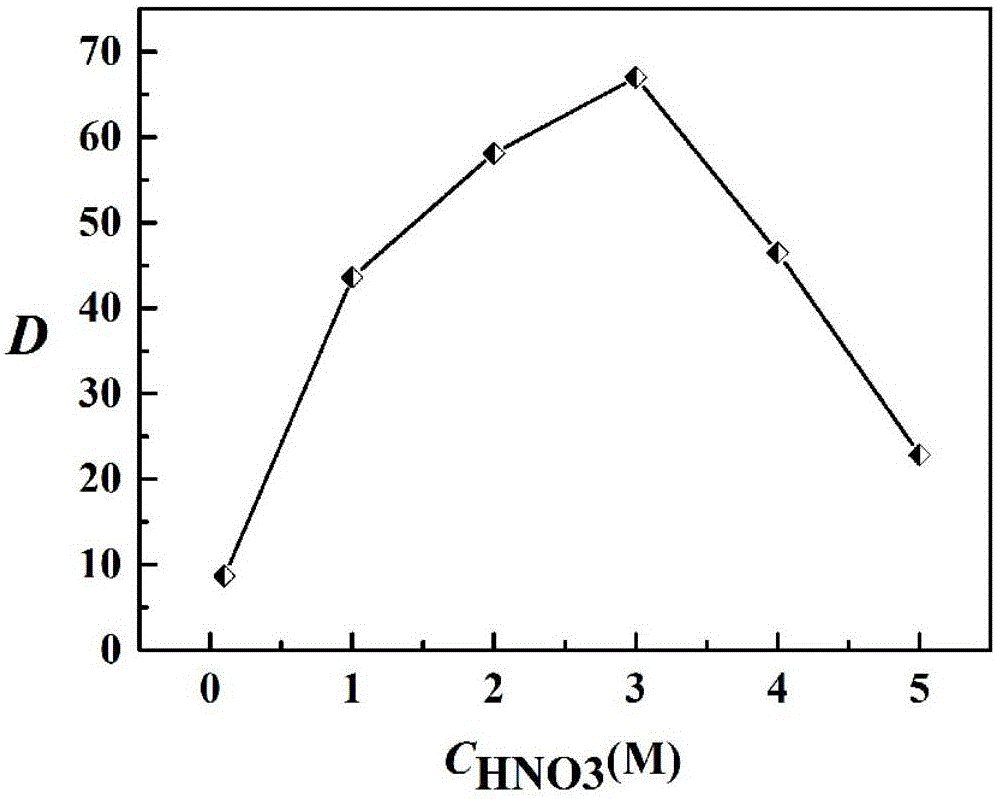
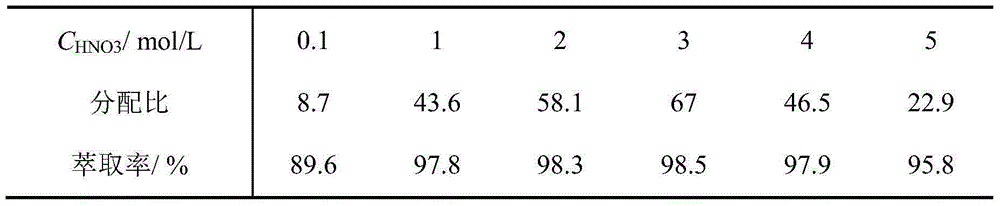
Image
Examples
Embodiment 1 1
[0026] Example 1 11,23-bis(propionamido)-25,27-bis(2-propoxy)-calix[4]arene-crown-6
[0027] 1) Synthesis of 11,23-bis(nitro)-25,27-bis(2-propoxy)-calix[4]arene-crown-6: 4.26g of 25,27 in 1,3 alternate conformation -Bis(2-propoxy)-calix[4]arene-crown-6 is dissolved in 150mL of acetic anhydride, cooled to -10°C, stirred for 30min, and mixed with 100mL of acetic anhydride, 100mL of acetic acid, and 1mL of concentrated nitric acid. Slowly add dropwise to the above system, continue stirring for 3 hours after the completion of the drop, warm to room temperature, and stir for 12 hours. Pour the reaction solution into 500 mL of ice-water mixture, stir until solid precipitation is complete, filter with suction, and dry. The crude product obtained is recrystallized with a mixed solvent of methanol and ether with a volume ratio of 5:2 to obtain a pale yellow powder solid. Namely 11,23-bis(nitro)-25,27-bis(2-propoxy)-calix[4]arene-crown-6, the yield=93%;
[0028] 2) The synthesis of 11,23-b...
Embodiment 2 1
[0033] Example two 11,23-bis(propionamido)-25,27-bis(2-propoxy)-calix[4]arene-crown-6
[0034] The method for synthesizing white flaky crystals 11,23-bis(amino)-25,27-bis(2-propoxy)-calix[4]arene-crown-6 is the same as in Example 1.
[0035] Add 0.740 g of the obtained white flake crystals to 20 mL of dichloromethane to dissolve, ventilate nitrogen, cool to -5° C., add 0.25 g of propionyl chloride, and add 0.3 g of triethylamine dropwise. After finishing the drop, heat to reflux for 3 hours. After the completion of the reaction, the reaction solution was directly spin-dried under reduced pressure, dissolved in dichloromethane, and washed three times with distilled water. The organic phases were combined, dried with sodium sulfate, filtered with suction, the filtrate was spin-dried, and a small amount of dichloromethane was added to dissolve. The white precipitate is obtained in petroleum ether, filtered and dried to obtain the product 11,23-bis(propionamido)25,27-bis(2-propoxy)-cal...
Embodiment 3 1
[0036] Example three 11,23-bis(butyramido)25,27-bis(2-propoxy)-calix[4]arene-crown-6
[0037] 1) Synthesis of 11,23-bis(nitro)-25,27-bis(2-propoxy)-calix[4]arene-crown-6: 4.26g of 25,27 in 1,3 alternate conformation -Bis(2-propoxy)-calix[4]arene-crown-6 is dissolved in 150mL of acetic anhydride, cooled to -15℃, stirred for 30min, and a mixture of 100mL of acetic anhydride, 100mL of acetic acid and 1mL of concentrated nitric acid Slowly add dropwise to the above system, continue stirring for 3 hours after the completion of the drop, warm to room temperature, and stir for 12 hours. The reaction solution was poured into 500 mL of ice-water mixture, stirred until solid precipitation was complete, filtered with suction, and dried. The crude product obtained was recrystallized with a mixed solvent of methanol and ether with a volume ratio of 5:2 to obtain a pale yellow solid.
[0038] 2) The synthesis of 11,23-bis(amino)-25,27-bis(2-propoxy)-calix[4]arene-crown-6: Take 0.8g of the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com