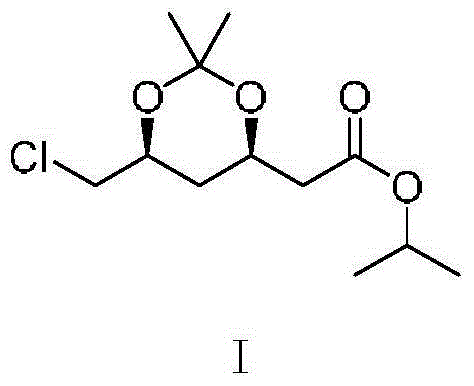
Preparation method of (4R-cis)-6-chloromethyl-2, 2-dimethyl-1, 3-dioxane-4-acetic acid isopropyl ester
A technology of isopropyl acetate and 4r-cis, applied in the field of biopharmaceuticals, can solve problems such as high cost, cumbersome steps, complicated post-processing, etc., and achieve the effects of safe reaction and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
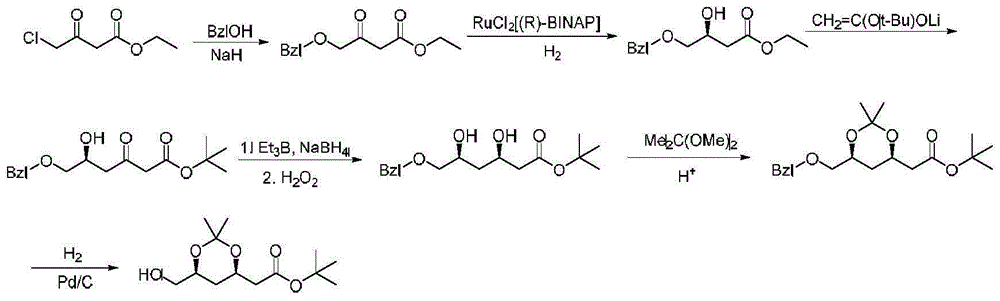
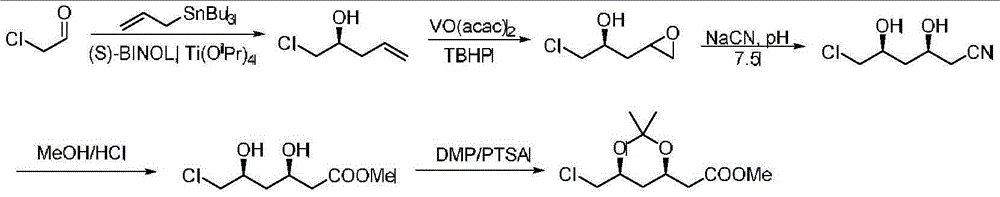
Image
Examples
Embodiment 1
[0036] Add 140ml of pH 6.5 0.1MPBS buffer solution into a 500ml reactor, add 2.55g of DERA enzyme (purchased from Suzhou Hanzyme, brand EW069, the same below) at 25°C, and then add mass 49.1 g of chloroacetaldehyde with a fraction of 24%, 13.7 g of acetaldehyde with a mass fraction of 99.6%, and 100 ml of deionized water are added to provide a reaction environment for DERA, and the addition is controlled within 3 hours, and the pH is controlled during the entire reaction process. Between 6.5 and 6.7, after the dropwise addition, continue to stir the reaction for 1 h until the reaction is complete, then add acetone to the system to remove the aldolase, then filter through diatomaceous earth, extract three times with ethyl acetate, combine the organic phases, dry and spin Evaporate to obtain 14g of light yellow oily liquid, which is compound ⅳ ((4R)-6-(chloromethyl)tetrahydro-2H-pyran-2,4-diol), its GC purity is 77%, single-step The resulting molar yield was 56%.
Embodiment 2
[0038] At a temperature of 25°C, add 2.55 g of DERA enzyme (purchased from Suzhou Hanzyme, brand EW069) and 140 ml of deionized water into the reactor, stir evenly, and add chloroacetaldehyde with a mass fraction of 24% by feeding 75g, 13.7g of acetaldehyde with a mass fraction of 99.6%. The pH is controlled between 6.5 and 6.7 during the entire reaction process. After the dropwise addition is completed, continue to stir the reaction for 1h until the reaction is complete, and then add acetone to the system to remove the aldolase. Filtration with diatomaceous earth, extraction with ethyl acetate three times, combined organic phases, drying, and rotary evaporation gave 15.8 g of light yellow oily liquid iv with a GC purity of 70% and a single-step molar yield of 61.2%.
Embodiment 3
[0040] At a temperature of 25°C, add 2.55g of DERA enzyme and 140ml of deionized water into the reactor, stir evenly, and add 49.1g of chloroacetaldehyde with a mass fraction of 24%, and acetaldehyde with a mass fraction of 99.6% 20g, the pH is controlled between 6.5 and 6.7 during the whole reaction process. After the dropwise addition, continue to stir the reaction for 1h until the reaction is complete, then add acetone to the system to remove the aldolase, and then filter through diatomaceous earth and extract with ethyl acetate. Three times, the combined organic phases were dried and rotary evaporated to obtain 14.5 g of light yellow oily liquid iv with a GC purity of 70% and a single-step molar yield of 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com