Electrolyte containing benzene dinitrile and lithium ion battery applying electrolyte
A technology of electrolyte and phthalonitrile, which is applied in the field of lithium battery preparation, can solve problems such as side reactions, achieve the effects of reducing decomposition, reducing gas production, and improving cycle and high temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the preparation method of the lithium ion battery of this embodiment, the coating area density is determined according to the capacity design (1640mAh) of the battery and the capacity of the positive and negative electrode materials. The positive active material was purchased from Hunan Shanshan lithium cobaltate material; the negative active material was purchased from Jiangxi Zichen Technology. The steps for preparing the positive electrode, preparing the negative electrode, preparing the electrolyte, preparing the separator, and assembling the battery are described as follows;
[0034] The steps of preparing the positive electrode are: mixing positive electrode active material lithium cobalt oxide, conductive carbon black and binder polyvinylidene fluoride in a mass ratio of 96.8:2.0:1.2, and dispersing in N-methyl-2-pyrrolidone to obtain Positive electrode slurry, uniformly coat the positive electrode slurry on both sides of the aluminum foil, dry, calender and vacu...
Embodiment 2~18
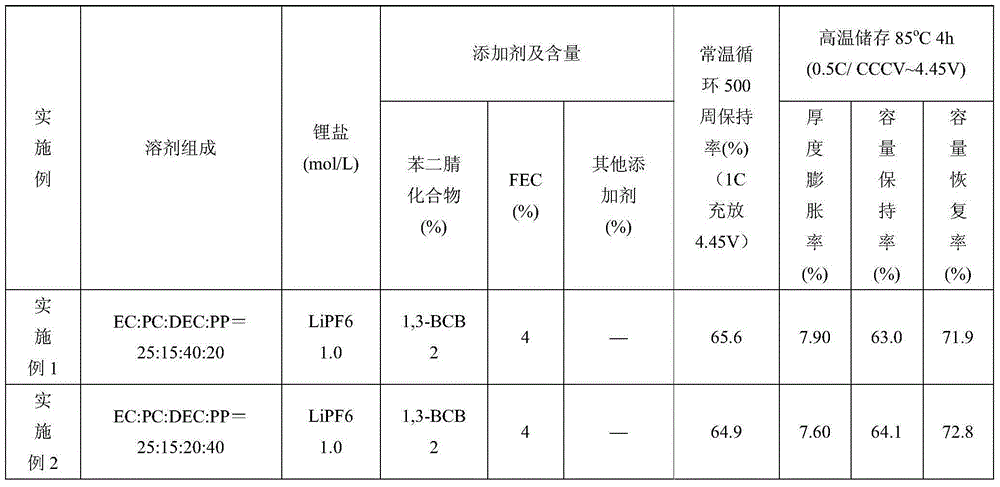
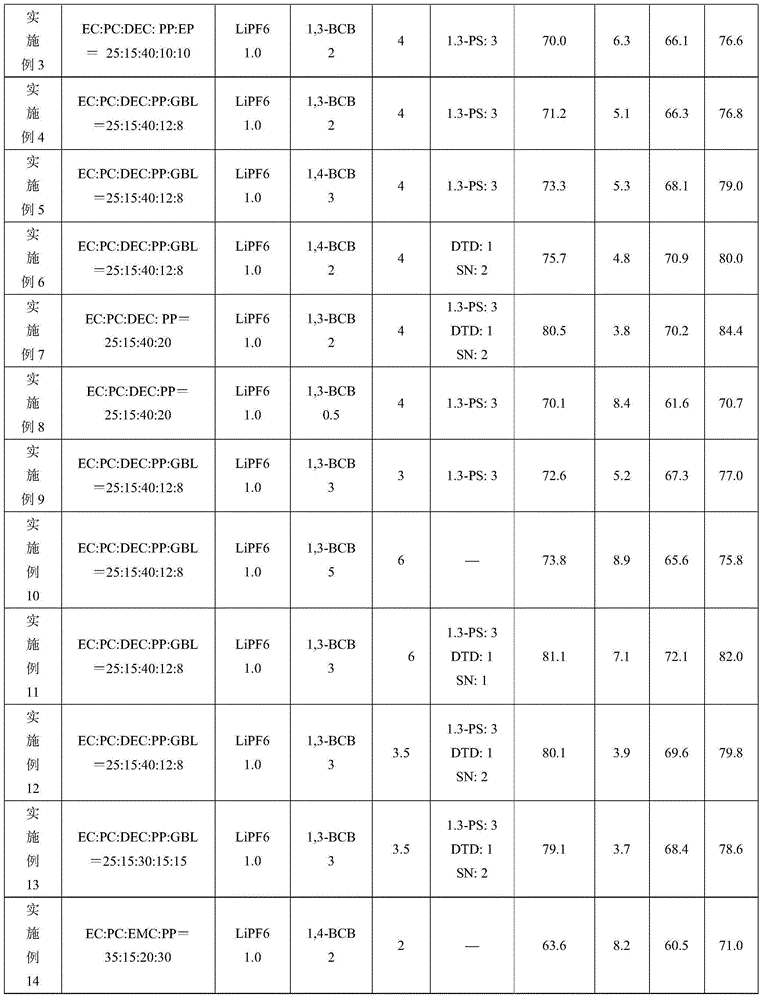
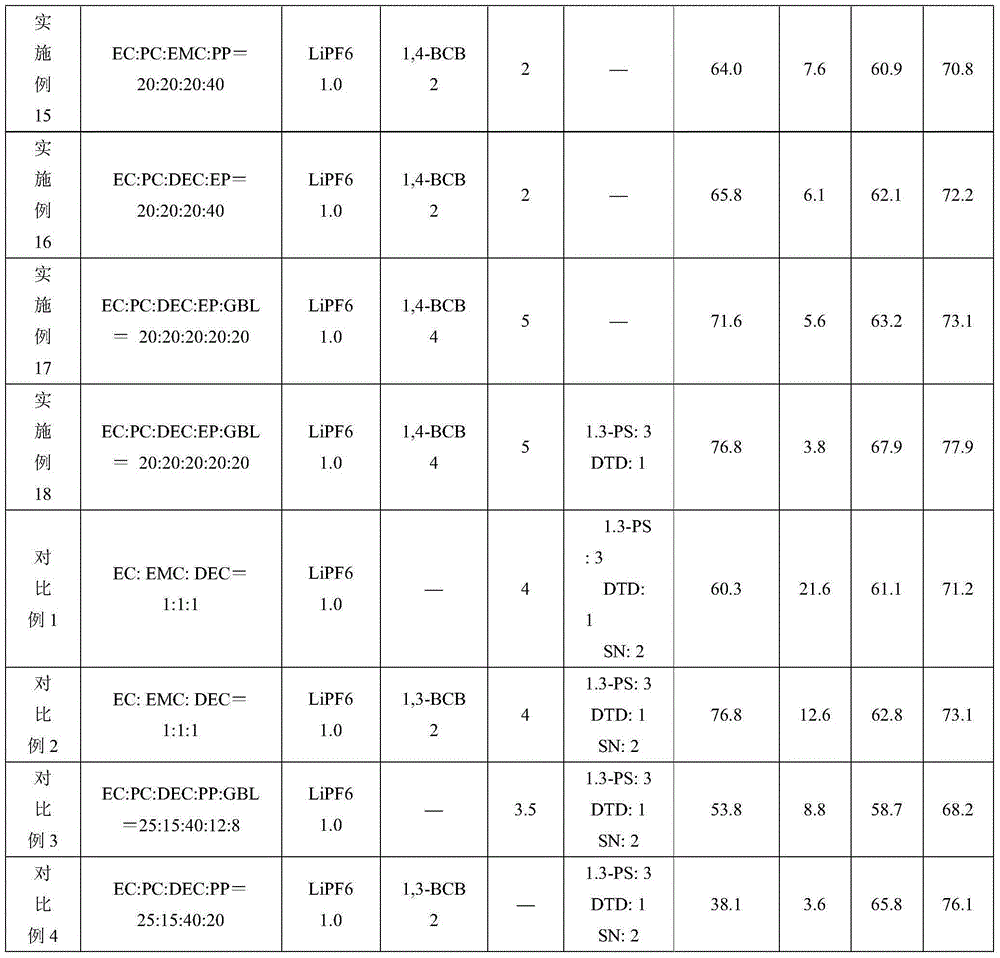
[0046] Examples 2 to 18 and Comparative Examples 1 to 4 are the same as Example 1, except that the solvent composition, additive composition and content (based on the total weight of the electrolyte) in the electrolyte are added as shown in Table 1. Table 1 shows the content of each component of the electrolyte additive and the battery performance test results. In the table, PP is propyl propionate, GBL is butyrolactone, EP is ethyl propionate, DTD is vinyl sulfate, 1,3-PS is 1,3-propane sultone, and SN is succinonitrile. 1,3-BCB is 1,3-benzenediacetonitrile, and 1,4-BCB is 1,4-benzenediacetonitrile.
[0047] Table 1
[0048]
[0049]
[0050]
Embodiment 7
[0051] Comparing Example 7 with Comparative Example 4 and Example 12 with Comparative Example 3 shows that:
[0052] In Comparative Example 3 without 1,3-benzenediacetonitrile, the capacity retention rate at the 500th cycle of 1C normal temperature cycle decreased from 80.5% to 53.8%. After storage at 85°C for 4 hours, the thickness expansion rate increased from 3.8% to 8.8%. It shows that a small amount of gas is produced, and its capacity retention rate and recovery rate are correspondingly reduced.
[0053] In Comparative Example 4 without fluoroethylene carbonate (FEC), the capacity retention rate at the 500th cycle of 1C normal temperature cycle decreased from 80.1% to 38.1%, indicating that the presence of fluoroethylene carbonate (FEC) can ensure that the battery has excellent Cycle performance.
[0054] Comparing Example 7 with Comparative Example 1 and Comparative Example 2, Comparative Examples 1 and 2 that do not contain carboxylate have obvious swelling when stored at hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com