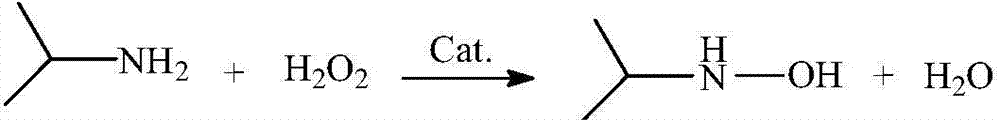The preparation method of n-isopropyl hydroxylamine
A technology of isopropyl hydroxylamine and isopropylamine, applied in the field of preparation of N-isopropyl hydroxylamine, can solve the problems of harsh catalyst synthesis conditions, troublesome carbon dioxide metering, difficulty in process amplification, etc., achieves good market space, and has low technical requirements for equipment , the effect of less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
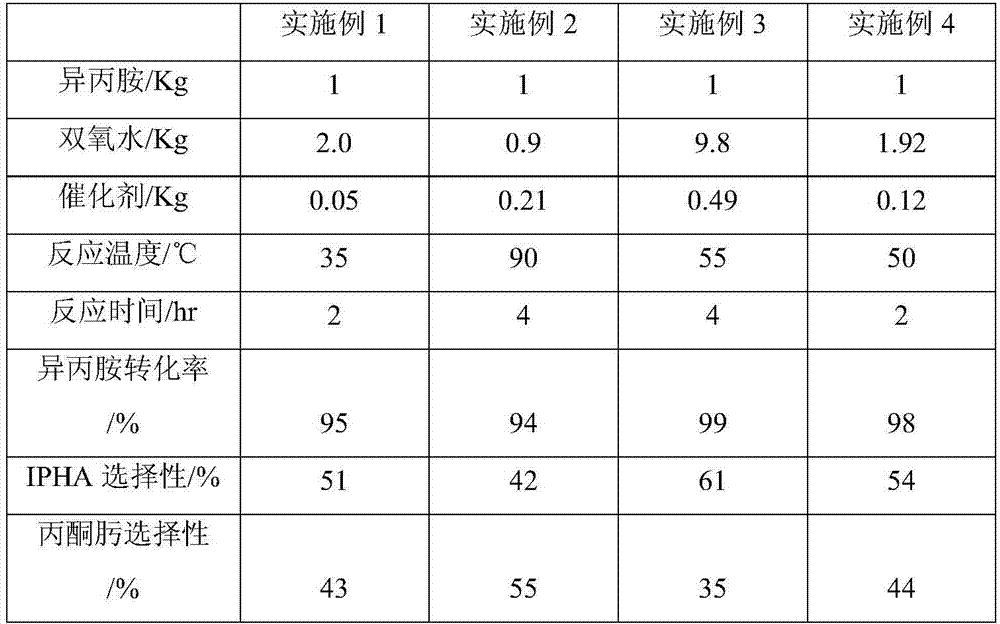
[0033] Pour 1 kg of isopropylamine and 2 kg of deionized water into the tank reactor equipped with 0.05 kg of titanium-silicon molecular sieve catalyst, start to feed cooling water into the reflux condenser of the reactor, stir and raise the temperature to 35°C under normal pressure, Start to drop 2.0Kg of 30% hydrogen peroxide into the reaction kettle, and control the dropping time at 2hr. After the hydrogen peroxide dripping is completed, continue the insulation reaction for 2hr, and the reaction ends. The reaction solution was sampled, and the components of the reaction solution were analyzed by gas chromatography. The molecular sieve catalyst in the reaction liquid is filtered out with a microfilter, and the obtained filtrate is sent to a rectification tower. Collect fractions at 33-45°C by rectification under normal pressure to recover the aqueous solution of isopropylamine, and then collect the aqueous solution of acetone oxime at 50-80°C under vacuum condition of 0.03MP...
Embodiment 2
[0037] Put the measured 1kg of isopropylamine and 2kg of deionized water into the tank reactor equipped with 0.21kg of titanium-silicon molecular sieve TS-1 catalyst, and start to feed cooling water into the reflux condenser of the reactor, and stir and heat up to At 90°C, start to drop 0.9Kg of 30% hydrogen peroxide into the reaction kettle, and the dropping time is controlled at 4hr. After the hydrogen peroxide drop is completed, continue to keep warm for 2hr, and the reaction ends. The reaction kettle was sampled, and the components of the reaction solution were analyzed by gas chromatography. The molecular sieve catalyst in the reaction liquid is filtered out with a microfilter, and the obtained filtrate is sent to a rectification tower. Collect fractions at 33-45°C by rectification under normal pressure to recover the aqueous solution of isopropylamine, and then collect the aqueous solution of acetone oxime at 50-80°C under vacuum condition of 0.03MPa. The still liquid a...
Embodiment 3
[0040] 9.8Kg of 30% hydrogen peroxide was added dropwise into the reaction kettle, the dropping time was controlled at 4hr, the addition amount of the titanium silicon molecular sieve catalyst was 0.49Kg, the reaction temperature was controlled at 55°C, and the rest were the same as in Example 2. The results of gas chromatography analysis are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com