Brefeldin A ester derivatives, and preparation and application thereof
A Brefeldin and Brefeldin technology, applied in drug combinations, antidote, antitumor drugs, etc., can solve the problems of low targeting, low bioavailability, poor water solubility, etc., and achieve good inhibitory activity. , the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
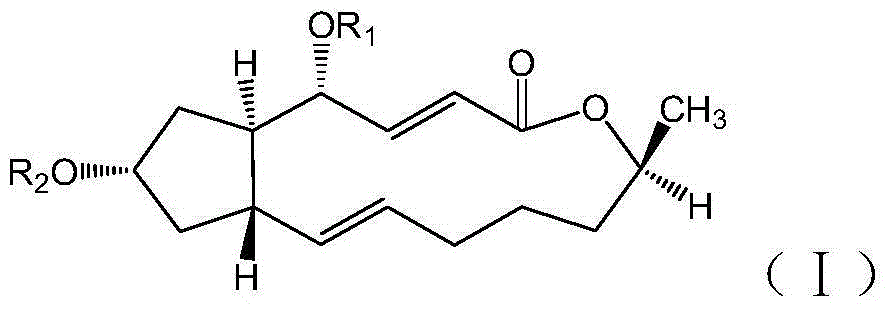
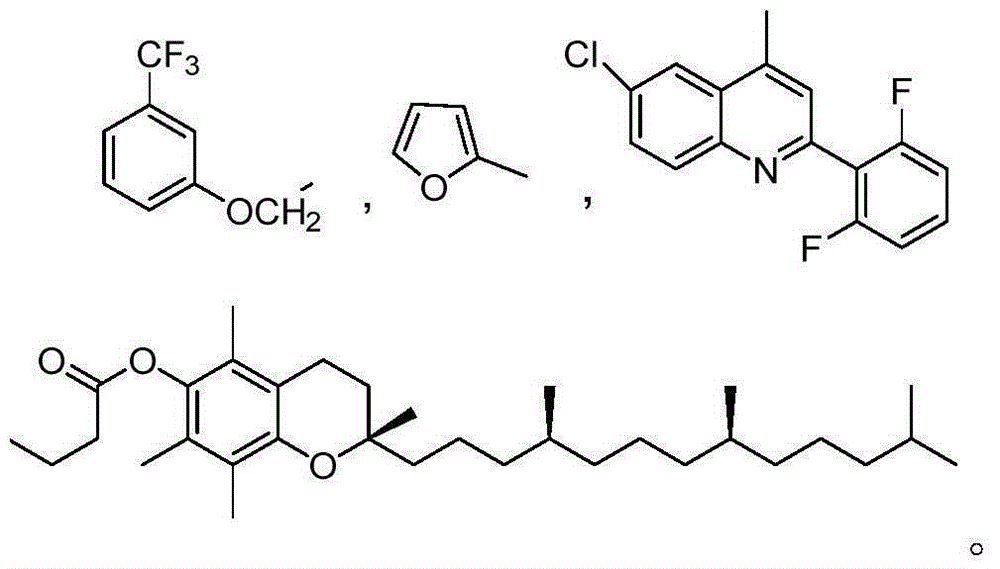
[0032] Example 1: Preparation of 4'7-2-(3-(trifluoromethyl)phenoxy)-brefeldin A diester acetate (I-1)
[0033] The reaction formula is as follows:
[0034]
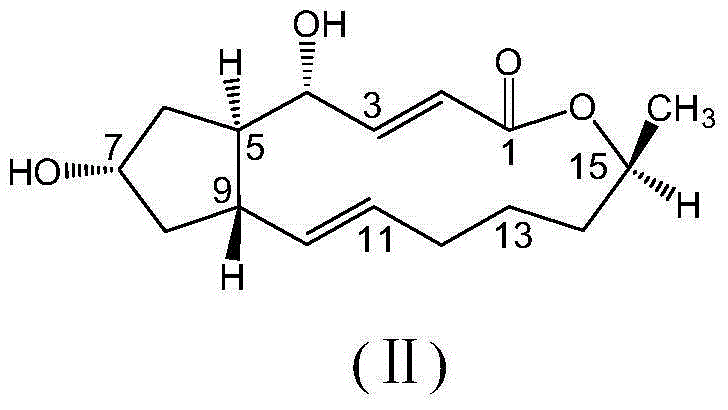
[0035] Weigh brefeldin A (Ⅱ) (1eq, 0.3mmol, 84mg), dissolve in 20mL of anhydrous dichloromethane to make (Ⅱ) solution, take 2-(3-(trifluoromethyl)phenoxy ) Acetic acid (Ⅲ-1) (4eq, 1.2mmol, 0.264g) was dissolved in 20ml of anhydrous dichloromethane to prepare (Ⅲ-1) solution, and (Ⅲ-1) solution was slowly added to (Ⅱ) solution Add EDC·HCl (4eq, 1.2mmol, 0.230g) and DMAP (3.3eq, 1mmol, 0.122g) in sequence, and nitrogen gas, microwave-assisted reaction at 44°C for 6.5h, the microwave power is 150W, the reaction process Whether the reaction was complete was detected by thin layer chromatography (ethyl acetate:petroleum ether=1:2, v / v). After the reaction, add 20ml of water to quench, then add 2×30ml of dichloromethane for extraction, wash the organic layer 2×50ml with water, and wash 2×50ml with saturated NaCl aqueous sol...
Embodiment 2
[0038] Example 2: Preparation of 7-2-furan-brefeldin A formic acid monoester (I-2) and 4' 7-2-furan-brefeldin A formic acid diester (I-3)
[0039] The reaction formula is as follows:
[0040]
[0041] Weigh brefeldin A (Ⅱ) (1eq, 0.3mmol, 84mg), dissolve in 20mL of anhydrous dichloromethane to make (Ⅱ) solution, take 2-furanoic acid (Ⅲ-2) (4eq, 1.2mmol , 0.135g) was dissolved in 20ml of anhydrous dichloromethane to make (Ⅲ-2) solution, and (Ⅲ-2) solution was slowly added to (Ⅱ) solution, and then EDC·HCl (4eq, 1.2 mmol, 0.230g) and DMAP (3.3eq, 1mmol, 0.122g), nitrogen flow, microwave reaction 7h under the condition of 44 ℃, microwave power is 150W, detects whether the reaction is complete with thin-layer chromatography in the reaction process (ethyl acetate : Petroleum ether=1:2, v / v). After the reaction was completed, 20ml of water was added to the reaction liquid to quench, and then 2×30ml of dichloromethane was added for extraction, the organic layer was washed with wa...
Embodiment 3
[0044] Example 3: 7-6-chloro-2-(2,6-difluorophenyl) quinoline-4-brefeldin A formic acid monoester (I-4) and 4'7-6-chloro- Preparation of 2-(2,6-difluorophenyl)quinoline-4-brefeldin A formic acid diester (Ⅰ-5)
[0045] The reaction formula is as follows:
[0046]
[0047] Weigh brefeldin A (II) (1eq, 0.3mmol, 84mg), dissolve it in 20mL of anhydrous dichloromethane to make a solution of formula (II), take 6-chloro-2-(2,6-difluoro Phenyl) quinoline-4-carboxylic acid (Ⅲ-3) (4eq, 1.2mmol, 0.3834g) was dissolved in 20ml of anhydrous dichloromethane to prepare formula (Ⅲ-3) solution, and formula (Ⅲ-3) The solution was slowly added to the solution of formula (II), and then EDC·HCl (4eq, 1.2mmol, 0.230g) and DMAP (3.3eq, 1mmol, 0.122g) were added in sequence, nitrogen gas was applied, and microwave reaction was carried out at 44°C for 7h. The power was 150W, and the completion of the reaction was detected by thin-layer chromatography during the reaction (ethyl acetate:petroleum et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com