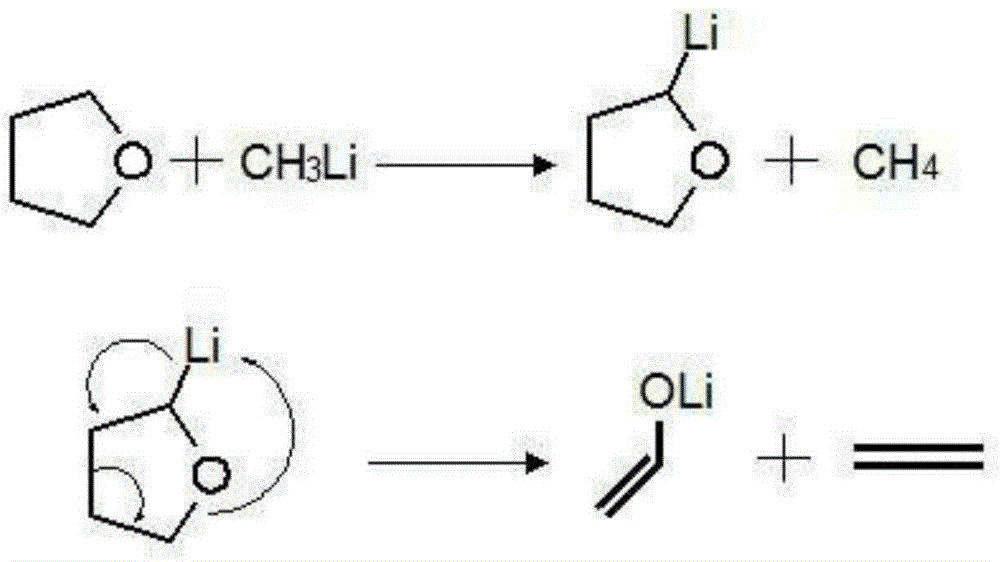
Methyllithium solution and preparation method thereof
A technology of methyl lithium and solution, which is applied in the field of preparation of methyl lithium solution, can solve the problems of comprehensive cost affecting product market competitiveness, high price of methyl tetrahydrofuran, and difficulty in solvent recovery and separation, so as to reduce production cost and improve safety High efficiency and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
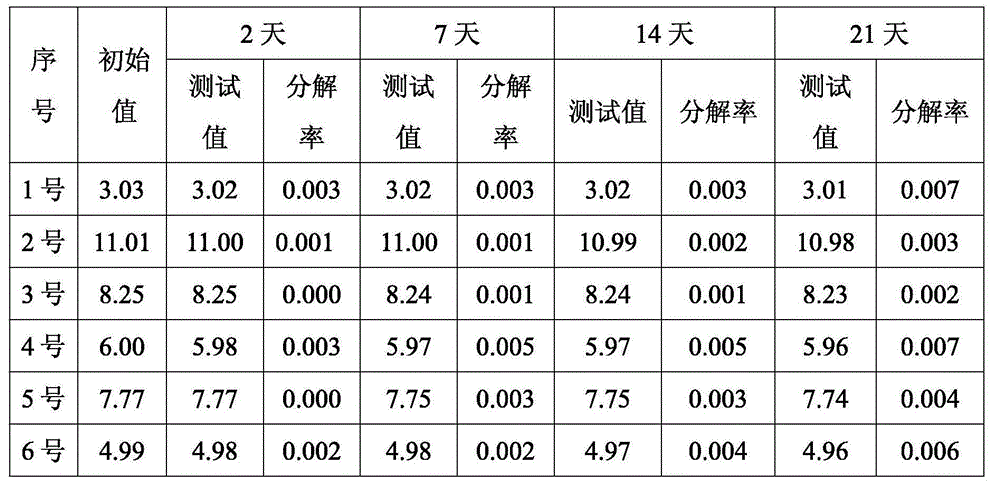
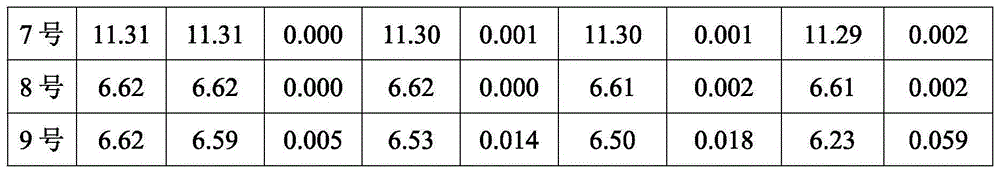
[0035] Under normal pressure at 20-40°C and under the protection of inert gas argon, 21g of metal lithium sand is wrapped with 50g of diethoxymethane and poured into a closed reactor, and the remaining 900g of diethoxymethane is mixed with 105g of lithium bromide and then added In the reactor, add 75g of methyl chloride into the reactor within 1 hour, and the reactor is cooled with a low-temperature refrigerant interlayer, and the temperature is controlled at 20-40°C. After the mixture is added, it is kept at 30-40°C for 1 hour, and the quality of methyllithium is obtained by filtration. A methyllithium solution with a fraction of 3.03% and a mass fraction of lithium bromide of 9.65% was named sample No. 1, and the yield of methyllithium was 98.9%.
Embodiment 2
[0037] Under normal pressure at 20-40°C and under the protection of inert gas argon, 63g of metal lithium sand was wrapped with 63g of diethoxymethane and poured into a closed reactor, 300g of lithium bromide was quickly added to metal lithium, and then the remaining 437g Diethoxymethane was added to the reactor, and 225g of methyl chloride was added to the reactor within 4 hours. The reactor was cooled with a low-temperature refrigerant interlayer, and the temperature was controlled at 20-40°C. After the mixture was added, it was kept at 30-40°C for 2 hours, and filtered A methyllithium solution with a mass fraction of methyllithium of 11.01% and a mass fraction of lithium bromide of 33.37% was obtained, named as sample No. 2, and the yield of methyllithium was 99.0%.
Embodiment 3
[0039]Under normal pressure at 20-40°C, under the protection of inert gas argon, 14g metal lithium sand is quickly added to reactor 1 under the protection of 140g diethoxymethane, keep reactor 1 airtight, and add 95g methyl bromide to reactor 1 within 1 hour , the reactor is cooled with a low-temperature refrigerant interlayer, and the temperature is controlled at 20-40°C. After the mixture is added, it is kept at 30-40°C for 2 hours to obtain a solution; at the same time, 7g of metal lithium sand is quickly added to the reaction with 140g of diethoxymethane. In the reactor 2, keep the reactor 2 airtight, add 25g of methyl chloride to the reactor 2 in 1 hour, cool the reactor with a low-temperature refrigerant interlayer, control the temperature at 20-40°C, and keep the temperature at 30-40°C for 2 hours after the addition of the mixture is completed. Obtain B solution; Filter A solution, B solution respectively, mix according to A solution: B solution=1: 1, obtain methyl lithi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com