Polypeptide block polymer and preparation method therefor and use thereof
A polymer and peptide block technology, applied in the fields of polymer chemistry and biomedical engineering, can solve the problems of lack of specificity, instability, killing normal cells, etc., and achieve good biocompatibility, low toxicity, and wide application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
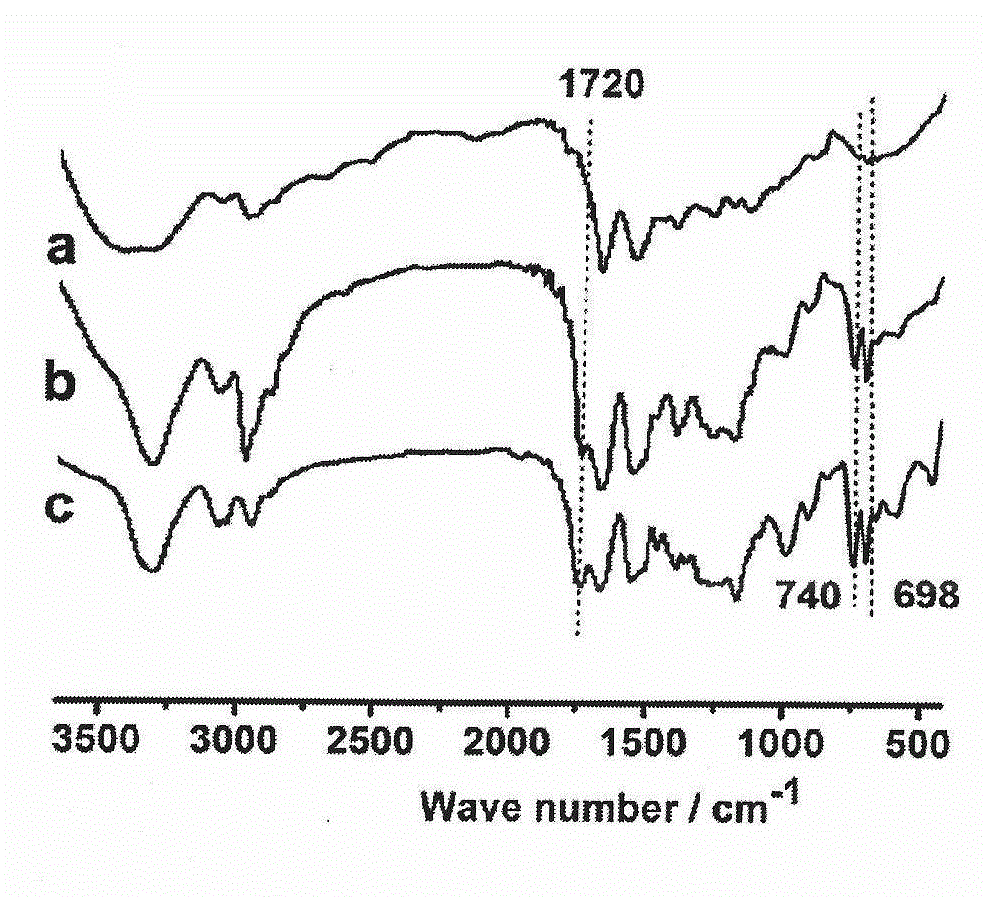
[0048] The synthesis of embodiment 1 polypeptide block polymer
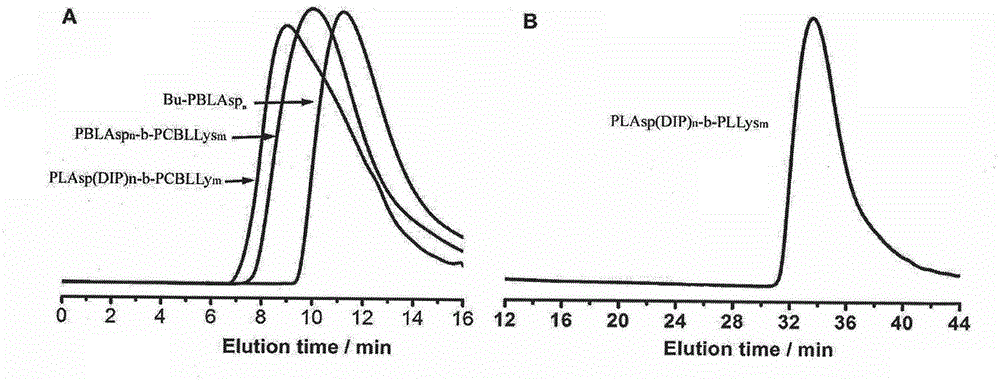
[0049] Polypeptide block polymer, the molecular formula is: PLAsp (DIP) n -b-PLLys m , the structural formula is:
[0050] Wherein, m=25, n=125.
[0051] PLAsp (DIP) n -b-PLLys m Synthetic steps:
[0052] (1) Synthesis of N-ε-benzyloxycarbonyl lysine benzyl ester, reaction mechanism and reaction process are as follows:
[0053]
[0054] Dissolve 18.25g (100mmol) of lysine hydrochloride and 8.0g (200mmol) of NaOH in 80ml of water to form a solution. Under the condition of 30°C, dissolve copper sulfate solution (12.5g of copper sulfate pentahydrate (50mmol) in 40ml of water) Add dropwise to the above solution; lower the reaction system to 0°C, under this condition, add 10gNaHCO 3 (120mmol), then dropwise excess 19ml (130mmol) of benzyl chloroformate, reacted for 3 hours at 0°C, overnight at room temperature; the N-Cbz-Lys-Cu in the reaction system 2+ The blue precipitate of the complex was collected, w...
Embodiment 2
[0076] Example 2 Preparation of blank vesicles and drug-loaded vesicles PALDOX
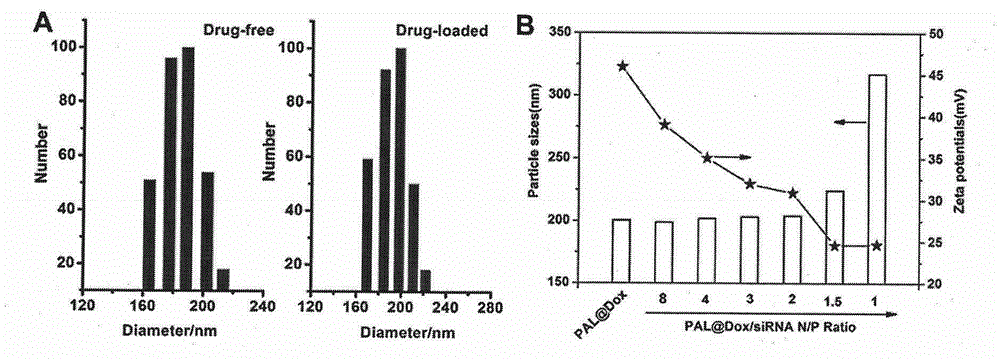
[0077] Preparation of blank vesicles: weigh 20 mg of the polypeptide block polymer PLAsp (DIP) in Example 1 n -b-PLLys m , dissolved with dilute hydrochloric acid (1%, mass concentration), the pH is about 2.0, stirred for 1h; within 20 minutes, the pH value was slowly adjusted to 5.0 with 0.5M NaOH aqueous solution; then within 20 minutes, with 0.05M NaOH aqueous solution The pH value was slowly adjusted to 6.4; finally, within 30 minutes, the pH value was slowly adjusted from 6.4 to 7.4 with 0.001 M NaOH aqueous solution, and then filtered through a 450 nm filter membrane to finally obtain a blank vesicle solution.
[0078] Drug-loaded vesicles (PALDOX) are prepared using the same method: weigh the polypeptide block polymer PLAsp (DIP) in Example 1 n -b-PLLys m 20 mg and 2 mg of doxorubicin hydrochloride were dissolved with dilute hydrochloric acid (1%, mass concentration), the pH was about 2....
Embodiment 3
[0080] Example 3 Preparation of drug-loaded vesicle PALsiRNA and combined drug-loaded vesicle PAL (DOX / siRNA)
[0081] A certain amount of siRNA (1 μg, purchased from Guangzhou Ruibo Biotechnology Co., Ltd.) was added to a known amount of blank vesicles and drug-loaded vesicles (PALDOX) solution in Example 2, according to the polypeptide block polymer PLAsp (DIP) n -b-PLLys m The molar ratios of amino groups and siRNA phosphate groups are 0.5:1, 1:1, 1.5:1, 2:1, 3:1, 4:1, and 8:1. Make up to the volume required for the experiment with deionized water, shake on a shaker for 30 minutes after mixing, and let stand for 10 minutes to obtain a series of drug-loaded vesicle PALsiRNA solutions and combined drug-loaded vesicle PAL ( DOX / siRNA) solution.
[0082] The particle size and surface potential of the drug-loaded vesicle PALDOX in Example 2 and the combined drug-loaded vesicle PAL (DOX / siRNA) in Example 3 were measured with a dynamic light scattering instrument (DLS), and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com