Catalyst and preparation method for preparing synthesis gas through reforming reaction of CO<2> and CH<4>
A technology for catalysts and synthesis gas, which is applied in chemical instruments and methods, inorganic chemistry, and bulk chemical production, etc., and can solve problems such as loss of active components, reduction of active sites, and deactivation of catalysts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step one: accurately weigh 41.295g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O, analytically pure), stirred and dispersed in 67.7304g acetone solution according to the solid-to-liquid ratio (1:5), and the concentration was 1.42mol L -1 The nickel salt dispersion liquid, the γ-Al after heat treatment at 500 ℃ 2 o 3 As a carrier, add it to the above-mentioned nickel salt dispersion according to the ratio of the mass ratio of the carrier to the impregnation solution of 1:5, and use ultrasonic strengthening treatment at 20-50°C for 10 minutes, and continue to stir, stand at room temperature for 12-20h and then filter , and then dried in a vacuum dryer at 120°C for 12-20h to obtain a nickel-based catalyst precursor, roasted at 500-800°C for 2h, and then reduced for 2h under a hydrogen atmosphere at 500-800°C to prepare Nickel-based part Ni / γ-Al of nickel-based catalyst 2 o 3 , that is, component I, where the Ni loading is 9.87%.
[0027] Step 2: A certain amount of coconut sh...
Embodiment 2
[0030] Step one: accurately weigh 14.7404g nickel acetate (Ni(CH 3 COO) 2 4H 2 O, analytically pure), stirred and dispersed in 75.2692g of methanol solution according to the solid-to-liquid ratio of 1:5, and the concentration was 0.6726mol L -1 The nickel salt dispersion liquid, next treatment method is the same as embodiment 1, makes the nickel base part Ni / γ-Al of catalyst 2 o 3 , as component I of the catalyst, where the loading of Ni is 5.07%.
[0031] Step 2: Except that biochar is selected for component II, other processes are the same as in Example 1.
[0032] Step 3: Same as Example 1.
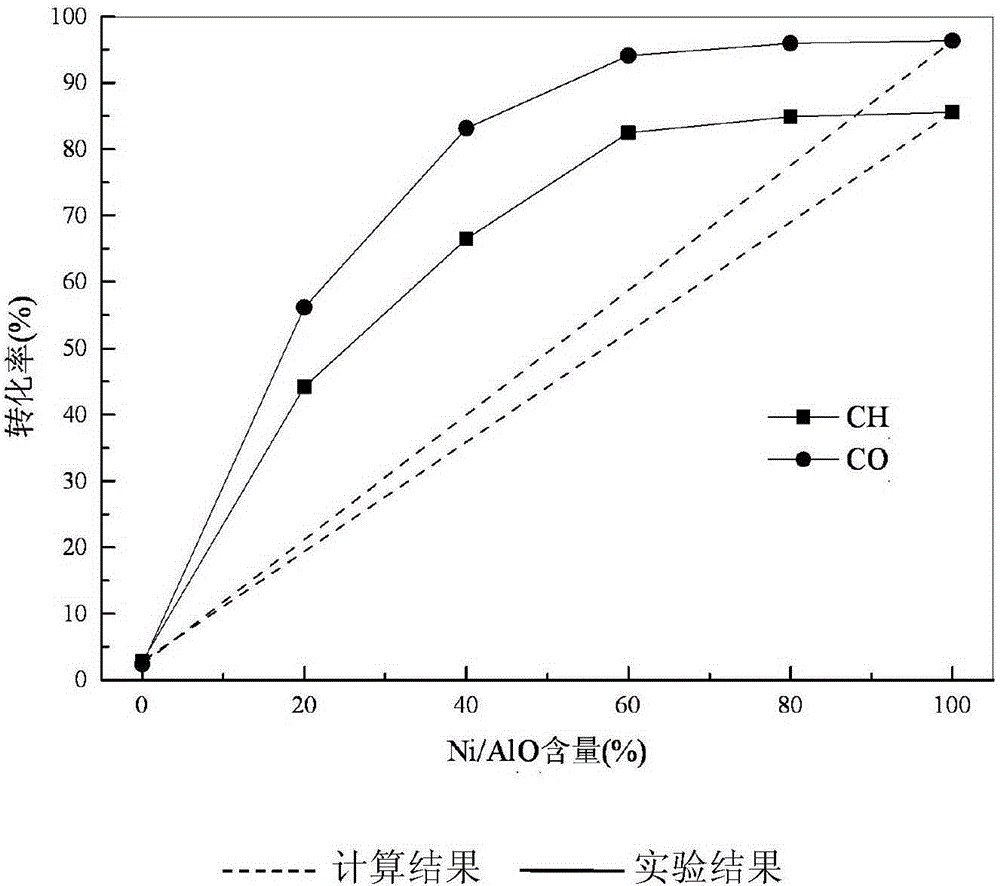
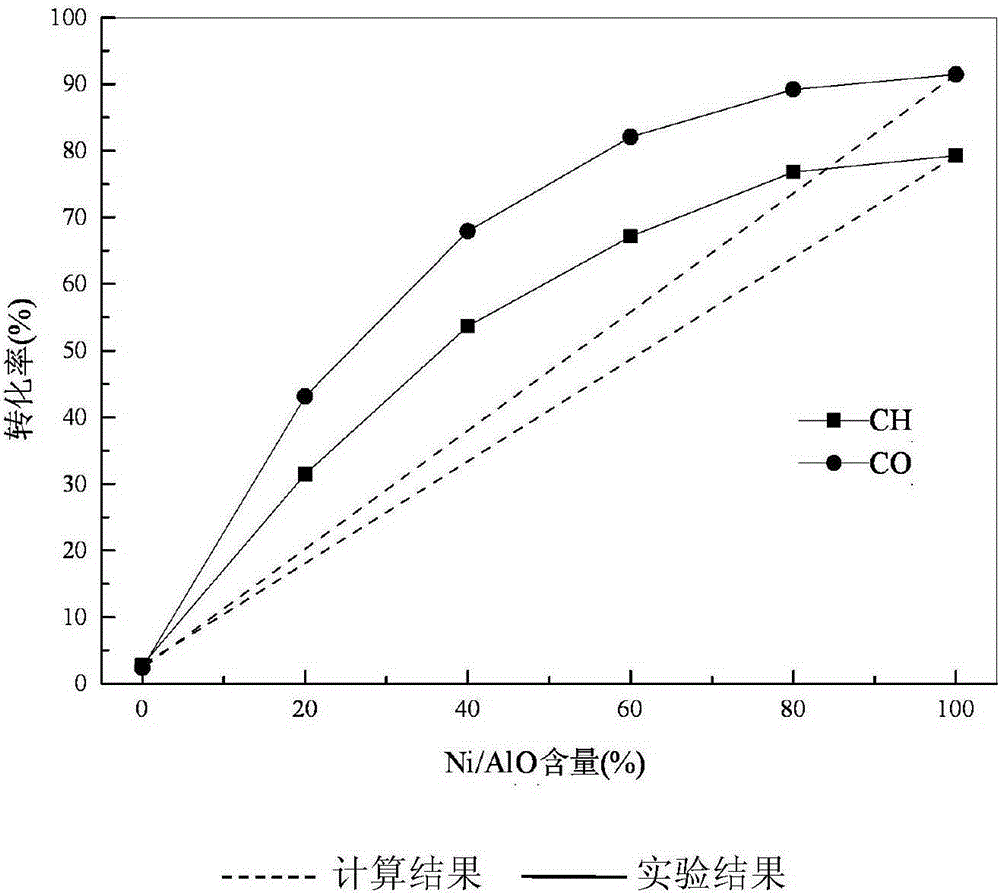
[0033] Reaction 5h obtains stable catalytic conversion rate, see figure 2 As shown, when Ni / γ-Al 2 o 3 When the content is 80%, CH 4 The conversion rate reached 76.81%, CO 2 The conversion rate reached 89.23%, while the theoretical CH 4 and CO 2 The conversions were 63.98% and 73.63%, respectively. The reforming reaction result of the catalyst of the present invention is ...
Embodiment 3
[0035] Step one: accurately weigh 3.7541g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O, analytically pure), was dispersed in 98.6057g of 20% water-ethanol mixed solution according to the solid-liquid ratio of 1:5, and the concentration was 0.1291mol L -1 The nickel salt dispersion liquid, next treatment method is the same as embodiment 1, makes the nickel base part Ni / γ-Al of catalyst 2 o 3 , as component I of the catalyst, where the loading of Ni is 1.24%.
[0036] Step 2: Except for the pyrolysis coke of lignite selected for component II, other processes are the same as in Embodiment 1.
[0037] Step 3: Except that the reaction temperature is 900°C, the reaction space velocity is 3600mL·g -1 h -1 (Under the standard state), other processes are the same as embodiment 1.
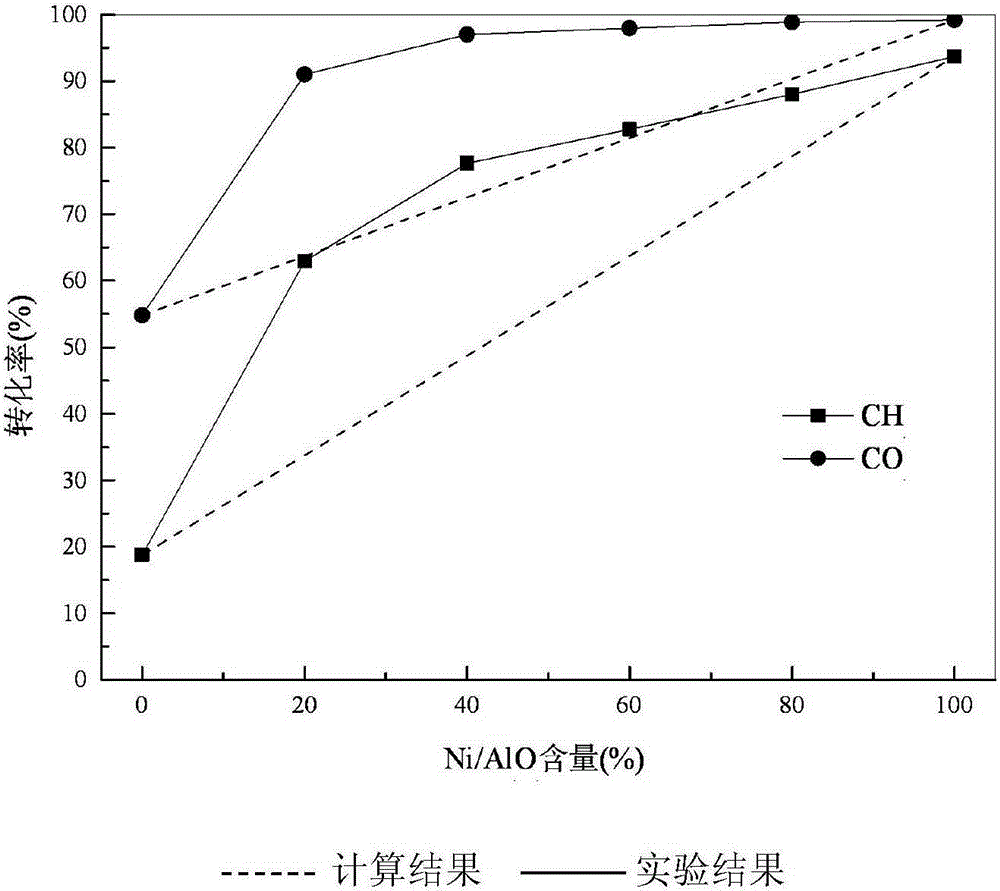
[0038] Reaction 5h obtains stable catalytic conversion rate, see image 3 As shown, when Ni / γ-Al 2 o 3 When the content is 60%, CH 4 The conversion rate reaches 82.75%, CO 2 The conversion rate reaches 97...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com