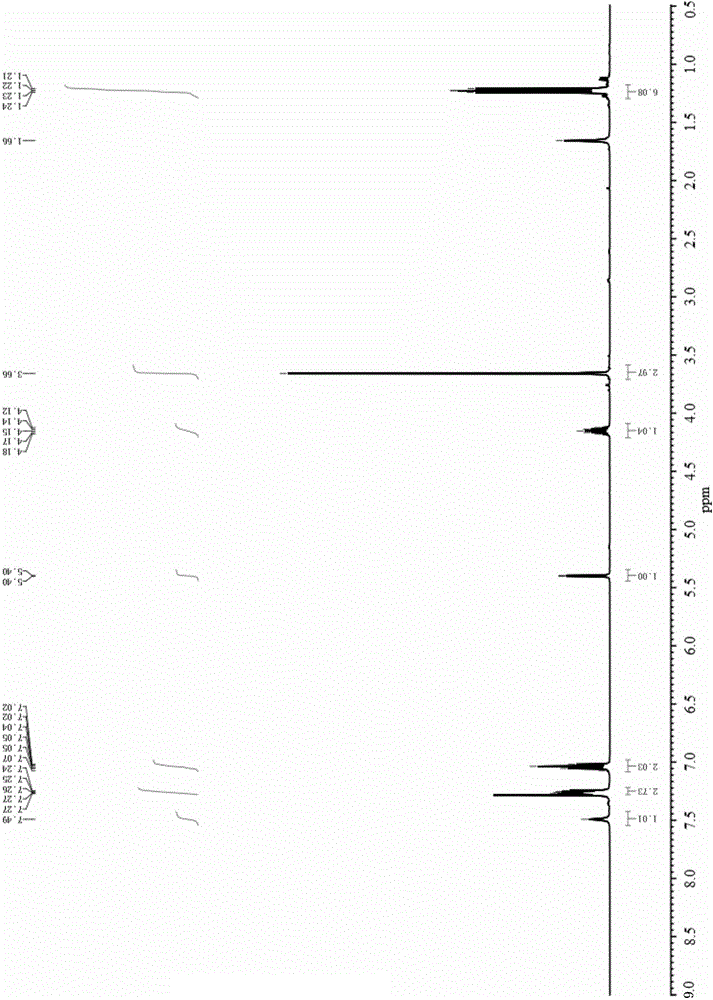
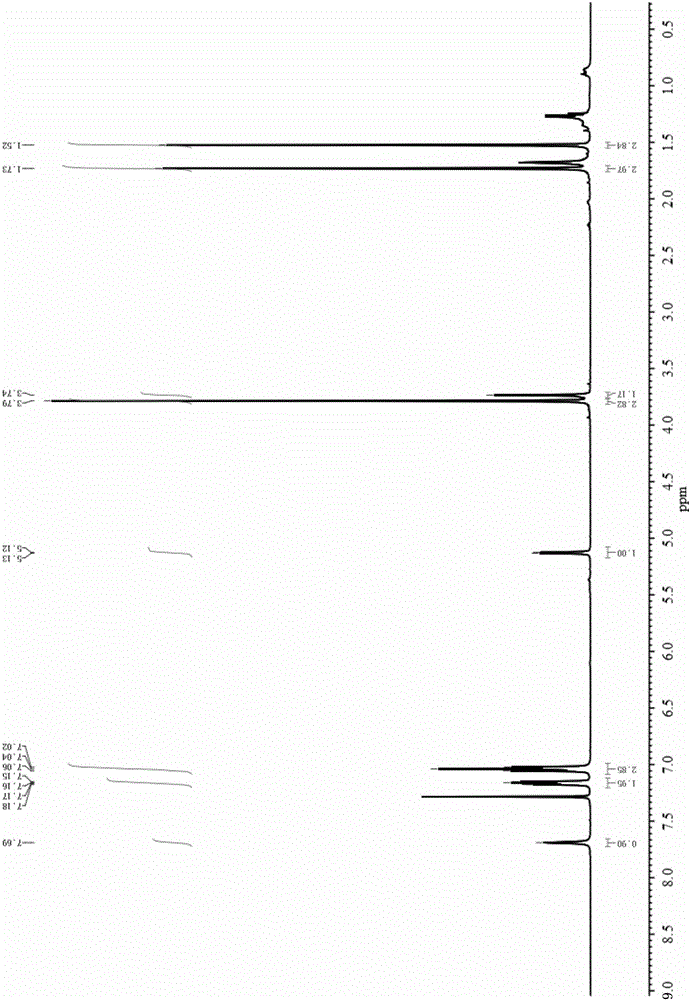
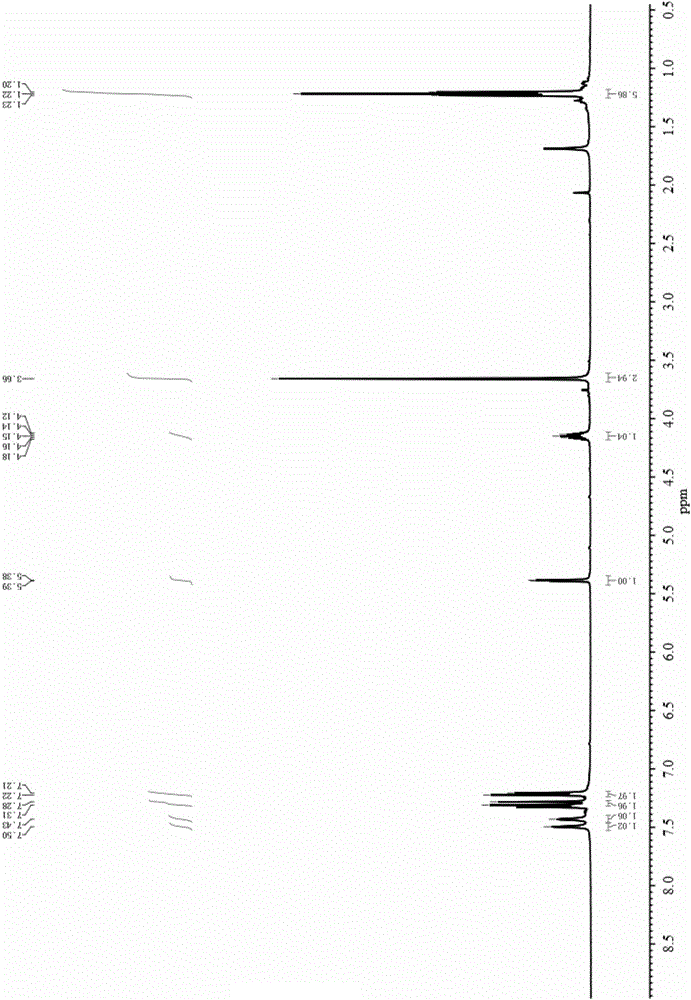
Method for preparing rosuvastatin midbody
A technology for rosuvastatin and intermediates, applied in the field of pharmaceutical intermediates, can solve the problems of expensive catalysts, low yields, long reaction times, etc., achieve good industrial production prospects, reduce production costs, and reduce the generation of by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this example, 4-fluorobenzaldehyde, methyl isobutyryl acetate and thiourea were used as substrates to confirm the activity of related catalysts in catalyzing the bigienlli reaction.
[0043] Add 4-fluorobenzaldehyde (5.0mmol), methyl isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and the corresponding catalyst (0.5mmol) into a three-necked flask equipped with a thermometer and a condenser. Solvent 20 mL of 1,4-dioxane was stirred and heated to 90°C. After 12 hours of reaction, the reaction solution was washed with cooling water, extracted with ethyl acetate, dried with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE=1:4) The rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-acetic acid methyl ester was isolated.
[0044] Table 1 shows the effect of different catalysts on the reaction
[0045]
[0046]
[0047]
Embodiment 2
[0049] In this example, 4-fluorobenzaldehyde, methyl isobutyryl acetate and thiourea were used as substrates, and magnesium chloride was used as a catalyst. The effect of solvent and temperature on the reaction activity was investigated.
[0050] Add 4-fluorobenzaldehyde (5.0mmol), methyl isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and magnesium chloride (0.5mmol) into a three-necked flask equipped with a thermometer and a condenser, and add the corresponding Solvent (20mL), stirred and warmed to the specified temperature, after 12h reaction, the reaction solution was washed with cold water, extracted with ethyl acetate, dried with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE=1 :4) The rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-methyl acetate was isolated and obtained.
[0051] Table 2 shows the influence of solvent and reaction temperature on the...
Embodiment 3
[0056] In this example, aromatic aldehydes, isobutyryl acetate and thiourea were used as substrates, magnesium chloride was used as a catalyst, and the stability of the reaction conditions was investigated by changing the R group of the substrate.
[0057] Add aromatic aldehyde (5.0mmol), isobutyryl acetate (6.0mmol), thiourea (7.5mmol) and magnesium chloride (0.5mmol) into a three-necked flask equipped with a thermometer and a condenser. The solvent 1,4-diox 20 mL of six rings, stirred and heated to 90°C, followed by TLC until the reaction was complete, the reaction solution was washed with cold water, extracted with ethyl acetate, dried with anhydrous magnesium sulfate, filtered, distilled under reduced pressure, and then subjected to column chromatography (EA / PE= 1:4) The rosuvastatin intermediate 4-(4-fluorophenyl)-6-isopropyl-2-thiocarbonyl-1,4-dihydropyrimidine-5-methyl acetate was isolated and obtained.
[0058] Table 3 shows the stability of the reaction conditions to diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com