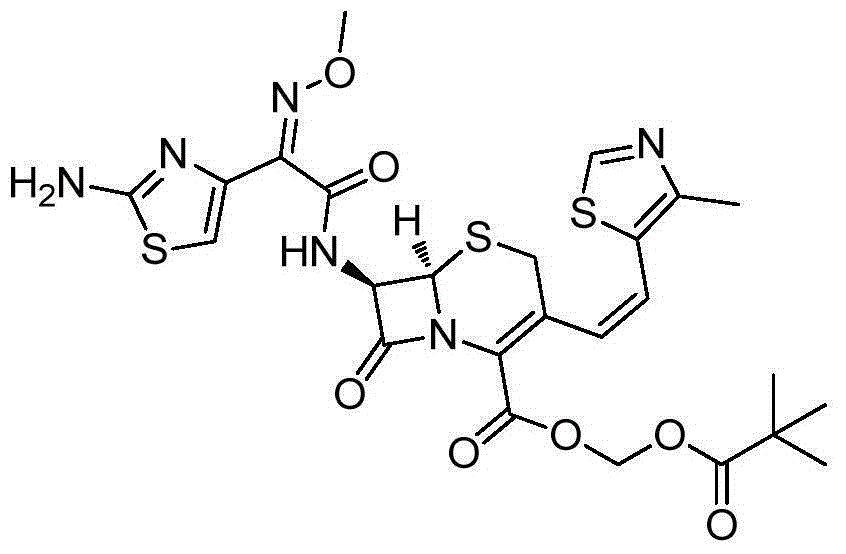
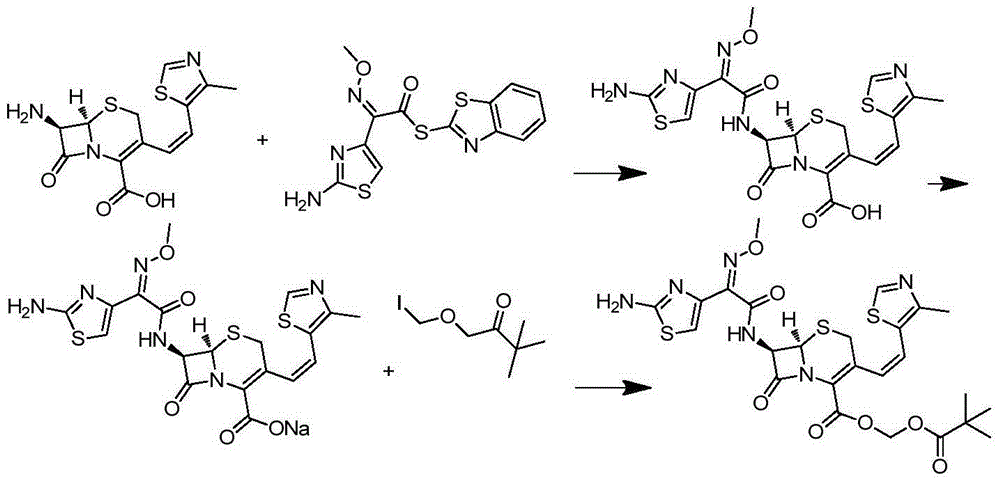
Preparation method of cefditore
A technology of cefditoren pivoxil and cefditoren pivoxil, which is applied in the field of preparation of cefditoren pivoxil, can solve the problems of increasing the amount of iodomethyl pivalate, large amount of iodomethyl pivalate, and large impurities , to achieve the effect of reducing the possibility of acylation and alkylation reactions, ingenious route design, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] At room temperature and protected from light, add 32.4g (0.10mol) of 7-ATCA and 37.6g (0.1075mol) of AE-active ester into 200ml of dichloromethane, add 1.0g of 4-dimethylaminopyridine, stir and cool down to 0°C ~5°C, slowly add 14.6g (0.145mol) triethylamine dropwise, after the addition, keep warm at 10°C~15°C for reaction, HPLC monitoring 7-ATCA1 HNMR (500MHz,D 2 O): 8.90 (1H, S), 6.75 (1H, S), 6.77 (1H, d, J = 11.8Hz), 6.32 (1H, d, J = 11.6Hz), 5.85 (dd, J 1 =4.8Hz,J 2 =8.0Hz,1H),5.26(d,J=4.8Hz,1H),3.83(3H,s),3.57(1H,d,J=18.6Hz),3.40(1H,d,J=18.6Hz), 2.34 (3H, s), 2.26–2.18 (m, 2H), 1.19 (d, J=6.4Hz, 12H, CH3).
[0028]Under room temperature dark conditions, add 60.8g (0.1mol) cefditoren acid diisopropylamine to 300ml N, N-dimethylformamide, then add 6.1g (10%) tetrabutylammonium bromide and sodium carbonate 5.3g (0.05mol), cooled to -15~-20°C, added 28.4g (0.1175) iodomethyl pivalate, and reacted at -15°C~-20°C, HPLC monitoring cefditoren sodium 3 The isomer conten...
Embodiment 2
[0030] Under the condition of avoiding light at room temperature, add 32.4g (0.10mol) of 7-ATCA and 38.0g (0.1085mol) of AE-active ester into 200ml of dichloromethane, add 1.0g of 4-dimethylaminopyridine, stir and cool down to 0°C ~5°C, slowly add 14.6g (0.145mol) triethylamine dropwise, after the addition, keep warm at 10°C~15°C for reaction, HPLC monitoring 7-ATCA<0.5% means the reaction is complete, after the reaction is completed, add 400ml to the reaction solution Pure water, stirred for 15 minutes, separated, collected the water phase, extracted once with dichloromethane, adjusted the pH to 3.5-4.4 with dilute hydrochloric acid, extracted three times with 1000ml ethyl acetate, washed once with water, and added to the ethyl acetate extract Add 3800mL of an aqueous solution containing 40g of diisopropylamine to the mixture, stir the reaction, and precipitate a solid at 5-10°C, filter and vacuum-dry to obtain 55.3g of a light yellow powdery solid, with a yield of 91.0%; the ...
Embodiment 3
[0033] Under the condition of avoiding light at room temperature, add 324g (1mol) 7-ATCA and 383g (1.095mol) AE-active ester into 2000ml dichloromethane, add 11g 4-dimethylaminopyridine, stir and cool down to 0℃~5℃, Slowly add 148g of triethylamine dropwise. After the addition, keep it warm at 10°C to 15°C for reaction. HPLC monitors that 7-ATCA<1.0% means the reaction is complete. After the reaction is complete, add 4000ml of pure water to the reaction solution, stir for 30min, and separate the liquid Collect the water phase, extract the water phase with dichloromethane once, adjust the pH to 3.5-4.4 with dilute hydrochloric acid, extract three times with 5L ethyl acetate, wash once with water, add an aqueous solution containing 350g diisopropylamine to the ethyl acetate extract 21 L, stirred and reacted, solid precipitated at 5-10°C, vacuum-dried by suction to obtain 560 g of light yellow powdery solid, yield 92.1; purity by HPLC was 99.24%, E isomer was not detected.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com