A low-temperature alkaline esterase estsl3 resistant to salt and organic solvents and its gene and application
A technology resistant to organic solvents and alkalinity, applied in the field of genetic engineering, can solve problems such as inability to apply, and achieve the effects of increasing the yield of the target product, reducing energy consumption, and improving flavor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Esterase gene EstSL3 clone
[0050] Extraction of genomic DNA of halophilic and alkalophilic bacteria: the bacterial genome extraction kit (DP302) of Tiangen Company was used to extract genomic DNA, and the specific operation was carried out in accordance with the instructions of the kit.
[0051] When we cloned the xylanase gene from this bacterium, we obtained the 3' end of the esterase gene. Sequence comparison analysis showed that the similarity of the gene was relatively low, and none of them had been characterized. Therefore, according to the sequence The nucleotide sequence of the 3' end of the esterase gene was designed, and three upstream TAIL-PCR specific primers were designed: the design direction was the direction of the unknown region to be amplified, the position of sp2 was designed to be inside sp1, and sp3 was located inside sp2. The distance between each two primers is not strictly regulated (for the convenience of electrophoresis identi...
Embodiment 2
[0055] Example 2 Preparation of recombinant esterase.
[0056] Introduced at the 5' and 3' ends of the gene, respectively NCOI and Hind III Restriction sites estSL3-m- F and estSL3-m- R is a pair of primers (see Table 1), and the genomic DNA of halophilic and alkalophilic bacteria is used as a template for PCR amplification. The PCR reaction parameters were: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 sec, annealing at 51°C for 30 sec, extension at 72°C for 1 min, 30 cycles, and incubation at 72°C for 5 min. The expression vector pET28a(+) was double digested ( NCOI + Hind III ), and the gene encoding esterase estSL3 double digestion ( NCOI + Hind III ), the cut gene fragment encoding mature esterase was connected to the expression vector pET28a(+) to obtain the esterase gene containing estSL3 The recombinant plasmid pET28a- estSL3 And transform Escherichia coli BL21 (DE3), obtain recombinant Escherichia coli strain BL21 / estSL3 .
...
Embodiment 3
[0058] Example 3 Determination of the properties of recombinant esterase EstSL3
[0059] 1. Activity analysis of recombinant esterase
[0060] The activity of recombinant esterase was determined by colorimetric method to determine the activity of esterase: the specific method is as follows: at pH 9.0, 30°C, 1 mL of reaction system included 100 μL of appropriate diluted enzyme solution, 20 μL of substrate (10 mM), add 880 μL 50mmol / L pH 9.0 phosphate buffer, react for 10 min and immediately set the OD 404 measure its absorbance. One enzyme activity unit (U) is defined as the amount of enzyme required to release 1 μmol p-nitrophenol per minute under given conditions.
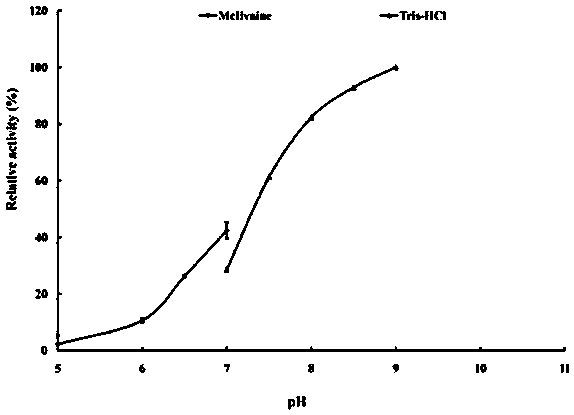
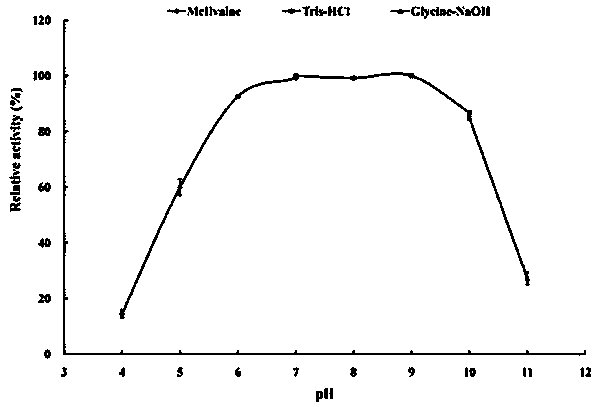
[0061] 2. Determination of optimum pH and pH stability of recombinant esterase EstSL3
[0062] Optimum pH determination: The purified recombinant esterase EstSL3 was subjected to an enzymatic reaction at 37°C in a 0.1M pH5.0–9.0 buffer. Determination of the pH stability of the enzyme: the enzyme solution was p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com